ULESFIA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Ulesfia, and what generic alternatives are available?
Ulesfia is a drug marketed by Shionogi Inc and is included in one NDA. There is one patent protecting this drug and one Paragraph IV challenge.
The generic ingredient in ULESFIA is benzyl alcohol. There are fifty drug master file entries for this compound. Additional details are available on the benzyl alcohol profile page.
DrugPatentWatch® Generic Entry Outlook for Ulesfia
Ulesfia was eligible for patent challenges on April 9, 2013.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 19, 2024. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for ULESFIA
| US Patents: | 1 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 221 |
| Clinical Trials: | 1 |
| Patent Applications: | 3,924 |
| Formulation / Manufacturing: | see details |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ULESFIA |
| What excipients (inactive ingredients) are in ULESFIA? | ULESFIA excipients list |
| DailyMed Link: | ULESFIA at DailyMed |
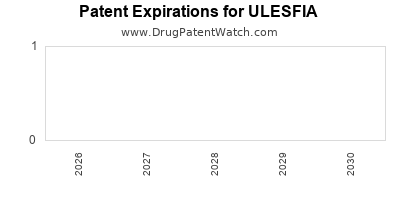
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ULESFIA
Generic Entry Date for ULESFIA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
LOTION;TOPICAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ULESFIA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Axis Clinical Trials | Phase 3 |
| Akorn, Inc. | Phase 3 |
| South Florida Family Health and Research Centers | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for ULESFIA
Paragraph IV (Patent) Challenges for ULESFIA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ULESFIA | Lotion | benzyl alcohol | 5% | 022129 | 1 | 2016-04-11 |
US Patents and Regulatory Information for ULESFIA
ULESFIA is protected by one US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ULESFIA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ULESFIA
Ectoparasite asphyxiator compositions and methods for their application
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF LICE INFESTATIONS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shionogi Inc | ULESFIA | benzyl alcohol | LOTION;TOPICAL | 022129-001 | Apr 9, 2009 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ULESFIA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Shionogi Inc | ULESFIA | benzyl alcohol | LOTION;TOPICAL | 022129-001 | Apr 9, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Shionogi Inc | ULESFIA | benzyl alcohol | LOTION;TOPICAL | 022129-001 | Apr 9, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Shionogi Inc | ULESFIA | benzyl alcohol | LOTION;TOPICAL | 022129-001 | Apr 9, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |


