TRUVADA Drug Patent Profile
✉ Email this page to a colleague
When do Truvada patents expire, and what generic alternatives are available?
Truvada is a drug marketed by Gilead and is included in one NDA. There are four patents protecting this drug and two Paragraph IV challenges.
This drug has sixty-two patent family members in twenty-nine countries.
The generic ingredient in TRUVADA is emtricitabine; tenofovir disoproxil fumarate. There are eighteen drug master file entries for this compound. Twenty-nine suppliers are listed for this compound. Additional details are available on the emtricitabine; tenofovir disoproxil fumarate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Truvada
A generic version of TRUVADA was approved as emtricitabine; tenofovir disoproxil fumarate by TEVA PHARMS USA on June 8th, 2017.
Summary for TRUVADA
| International Patents: | 62 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 114 |
| Clinical Trials: | 227 |
| Formulation / Manufacturing: | see details |
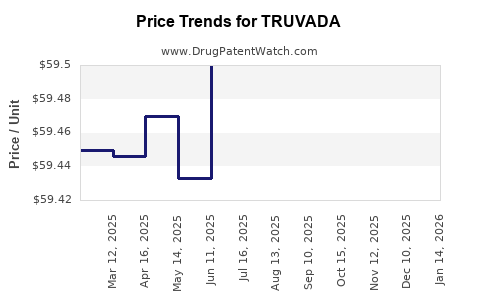
| Drug Prices: | Drug price information for TRUVADA |
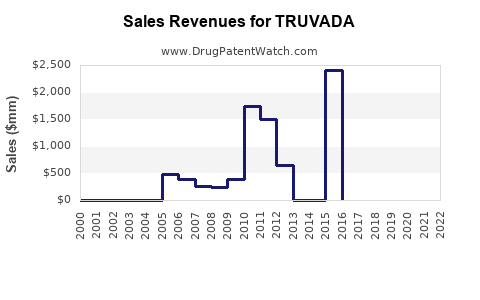
| Drug Sales Revenues: | Drug sales revenues for TRUVADA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for TRUVADA |
| What excipients (inactive ingredients) are in TRUVADA? | TRUVADA excipients list |
| DailyMed Link: | TRUVADA at DailyMed |
Recent Clinical Trials for TRUVADA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Assistance Publique - Hôpitaux de Paris, FRANCE | Phase 3 |
| Ministry of Health, Thailand | Phase 3 |
| Chiang Mai University, Thailand | Phase 3 |
Pharmacology for TRUVADA
Anatomical Therapeutic Chemical (ATC) Classes for TRUVADA
Paragraph IV (Patent) Challenges for TRUVADA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| TRUVADA | Tablets | emtricitabine; tenofovir disoproxil fumarate | 100 mg/150 mg 133 mg/200 mg 167 mg/250 mg | 021752 | 1 | 2017-05-19 |
| TRUVADA | Tablets | emtricitabine; tenofovir disoproxil fumarate | 200 mg/300 mg | 021752 | 1 | 2008-09-26 |
US Patents and Regulatory Information for TRUVADA
TRUVADA is protected by six US patents.
Patents protecting TRUVADA
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATMENT OF ADULTS INFECTED WITH HIV-1
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV-1 INFECTION IN PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV INFECTION
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV INFECTION
Compositions and methods for combination antiviral therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF HIV INFECTION
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-002 | Mar 10, 2016 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-001 | Aug 2, 2004 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-003 | Mar 10, 2016 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-004 | Mar 10, 2016 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TRUVADA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-004 | Mar 10, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-004 | Mar 10, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-003 | Mar 10, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| Gilead | TRUVADA | emtricitabine; tenofovir disoproxil fumarate | TABLET;ORAL | 021752-002 | Mar 10, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TRUVADA
See the table below for patents covering TRUVADA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 713374 | ⤷ Try a Trial | |
| Slovenia | 1243590 | ⤷ Try a Trial | |
| Ukraine | 81797 | ЛЕКАРСТВЕННАЯ ФОРМА ТЕНОФОВИРА ДИЗОПРОКСИЛ ФУМАРАТА И ЭМТРИЦИТАБИНА ДЛЯ КОМБИНИРОВАННОЙ АНТИВИРУСНОЙ ТЕРАПИИ;ЛІКАРСЬКА ФОРМА ТЕНОФОВІРУ ДИЗОПРОКСИЛ ФУМАРАТУ ТА ЕМТРИЦИТАБІНУ ДЛЯ КОМБІНОВАНОЇ АНТИВІРУСНОЇ ТЕРАПІЇ (PHARMACEUTICAL DOSAGE OF TENOFOVIR DISOPROXIL FUMARATE AND EMTRICITABINE FOR COMBINATION ANTIVIRAL THERAPY) | ⤷ Try a Trial |
| China | 1084745 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for TRUVADA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0513200 | 7/2004 | Austria | ⤷ Try a Trial | PRODUCT NAME: EMTRICITABIN; NAT. REGISTRATION NO/DATE: EU/1/03/261/001- EU/1/03/261/003 20031024; FIRST REGISTRATION: EU EU/1/03/261/003 |
| 0915894 | SPC/GB08/033 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TENOFOVIR DISOPROXIL AND THE SALTS (IN PARTICULAR THE FUMARATE), HYDRATES, TAUTOMERS AND SOLVATES THEREOF, TOGETHER WITH EMTRICITABINE AND EFAVIRENZ; REGISTERED: UK EU/1/07/430/001 20071213; REASON FOR LAPSE: SURRENDERED |
| 0915894 | SPC/GB05/041 | United Kingdom | ⤷ Try a Trial | SUPPLEMENTARY PROTECTION CERTIFICATE NO SPC/GB05/041 GRANTED TO GILEAD SCIENCES, INC. IN RESPECT OF THE PRODUCT COMPOSITION CONTAINING BOTH TENOFOVIR DISOPROXIL, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE, TAUTOMER OR SOLVATE THEREOF, TOGETHER WITH EMTRICITABINE , THE GRANT OF WHICH WAS ADVERTISED IN JOURNAL NO 6233 DATED 05/11/2008 HAS HAD ITS MAXIMUM PERIOD OF DURATION CORRECTED, SUBJECT TO THE PAYMENT OF THE PRESCRIBED FEES IT WILL EXPIRE ON 23/02/2020. |
| 0513200 | C00513200/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: EMTRICITABINE; REGISTRATION NUMBER/DATE: SWISSMEDIC 56880 25.10.2004 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


