SYMPROIC Drug Patent Profile
✉ Email this page to a colleague
When do Symproic patents expire, and what generic alternatives are available?
Symproic is a drug marketed by Bdsi and is included in one NDA. There are four patents protecting this drug.
This drug has seventy-four patent family members in twenty-seven countries.
The generic ingredient in SYMPROIC is naldemedine tosylate. One supplier is listed for this compound. Additional details are available on the naldemedine tosylate profile page.
DrugPatentWatch® Generic Entry Outlook for Symproic
Symproic was eligible for patent challenges on March 23, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be May 13, 2033. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for SYMPROIC
| International Patents: | 74 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 3 |
| Patent Applications: | 86 |
| Drug Prices: | Drug price information for SYMPROIC |
| What excipients (inactive ingredients) are in SYMPROIC? | SYMPROIC excipients list |
| DailyMed Link: | SYMPROIC at DailyMed |
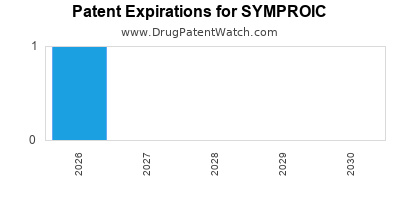
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for SYMPROIC
Generic Entry Date for SYMPROIC*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for SYMPROIC
| Drug Class | Opioid Antagonist |
| Mechanism of Action | Opioid Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for SYMPROIC
US Patents and Regulatory Information for SYMPROIC
SYMPROIC is protected by four US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of SYMPROIC is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting SYMPROIC
Preparation containing 6,7-unsaturated-7-carbamoyl morphinan derivatives
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystal of 6,7-unsaturated-7-carbamoyl morphinan derivative and method for producing the same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
6,7-unsaturated-7-carbamoyl substituted morphinan derivative
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
6,7-unsaturated-7-carbamoyl substituted morphinan derivative
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF OPIOID-INDUCED CONSTIPATION
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Bdsi | SYMPROIC | naldemedine tosylate | TABLET;ORAL | 208854-001 | Mar 23, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for SYMPROIC
When does loss-of-exclusivity occur for SYMPROIC?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Canada
Patent: 73961
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 51075
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 51075
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 2013172297
Estimated Expiration: ⤷ Try a Trial
Patent: 18219
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 51075
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 03149
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 1350120
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering SYMPROIC around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 100912782 | ⤷ Try a Trial | |
| Mexico | 348228 | CRISTAL DE DERIVADOS DE 6,7-INSATURADO-7-CARBAMOIL MORFINANO Y METODO PARA PRODUCIR EL MISMO. (CRYSTALLINE 6,7-UNSATURATED-7-CARBAMOYL MORPHINANE DERIVATIVE, AND METHOD FOR PRODUCING SAME.) | ⤷ Try a Trial |
| Portugal | 2639234 | ⤷ Try a Trial | |
| Portugal | 1889848 | ⤷ Try a Trial | |
| Japan | 5191574 | ⤷ Try a Trial | |
| Brazil | PI0610343 | composto derivado de morfinan substituìdo por 7-carbamoìla 6,7-insaturada, composição farmacêutica e uso do mesmo | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for SYMPROIC
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1889848 | 19C1042 | France | ⤷ Try a Trial | PRODUCT NAME: NALDEMEDINE OU L'UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES OU SOLVATES, EN PARTICULIER LE SEL DE TOSYLATE; REGISTRATION NO/DATE: EU/1/18/1291 20190220 |
| 1889848 | C 2019 033 | Romania | ⤷ Try a Trial | PRODUCT NAME: NALDEMEDINA SAU O SARE SAU SOLVAT ACCEPTABILE FARMACEUTIC; NATIONAL AUTHORISATION NUMBER: EU/1/18/1291; DATE OF NATIONAL AUTHORISATION: 20190218; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/18/1291; DATE OF FIRST AUTHORISATION IN EEA: 20190218 |
| 1889848 | 2019C/533 | Belgium | ⤷ Try a Trial | PRODUCT NAME: NALMEDINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF SOLVAAT HIERVAN, IN BIJZONDER HET TOSYLAATZOUT; AUTHORISATION NUMBER AND DATE: EU/1/18/1291 20190220 |
| 1889848 | CA 2019 00035 | Denmark | ⤷ Try a Trial | PRODUCT NAME: NALDEMEDIN ELLER ET FARMACEUTISK ACCEPTABELT SALT ELLER SOLVAT DERAF, SAERLIGT TOSYLAT SALTET; REG. NO/DATE: EU/1/18/1291 20190220 |
| 1889848 | 2019/038 | Ireland | ⤷ Try a Trial | PRODUCT NAME: NALDEMEDINE OR A PHARMACEUTICALLY ACCEPTABLE SALT OR SOLVATE THEREOF, IN PARTICULAR THE TOSYLATE SALT.; REGISTRATION NO/DATE: EU/1/18/1291 20190218 |
| 1889848 | 38/2019 | Austria | ⤷ Try a Trial | PRODUCT NAME: NALDEMEDINE ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ ODER SOLVAT DAVON, INSBESONDERE DAS TOSYLATSALZ; REGISTRATION NO/DATE: EU/1/18/1291 (MITTEILUNG) 20190220 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


