ROZLYTREK Drug Patent Profile
✉ Email this page to a colleague
When do Rozlytrek patents expire, and what generic alternatives are available?
Rozlytrek is a drug marketed by Genentech Inc and is included in two NDAs. There are fourteen patents protecting this drug.
This drug has one hundred and twenty-two patent family members in thirty countries.
The generic ingredient in ROZLYTREK is entrectinib. One supplier is listed for this compound. Additional details are available on the entrectinib profile page.
DrugPatentWatch® Generic Entry Outlook for Rozlytrek
Rozlytrek was eligible for patent challenges on August 15, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be October 20, 2030. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for ROZLYTREK
| International Patents: | 122 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 59 |
| Clinical Trials: | 9 |
| Patent Applications: | 473 |
| Drug Prices: | Drug price information for ROZLYTREK |
| What excipients (inactive ingredients) are in ROZLYTREK? | ROZLYTREK excipients list |
| DailyMed Link: | ROZLYTREK at DailyMed |
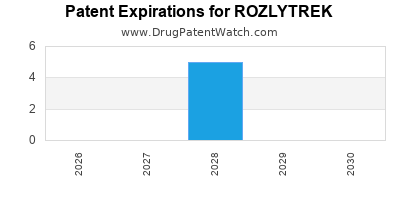

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ROZLYTREK
Generic Entry Dates for ROZLYTREK*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
Generic Entry Dates for ROZLYTREK*:
Constraining patent/regulatory exclusivity:
TREATMENT OF PEDIATRIC PATIENTS OLDER THAN 1 MONTH UP TO 12 YEARS OF AGE WITH SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION, AS DETECTED BY AN FDA-APPROVED TEST WITHOUT A KNOWN ACQUIRED RESISTANCE MUTATION, ARE METASTATIC OR WHERE SURGICAL RESECTION IS LIKELY TO RESULT IN SEVERE MORBIDITY, AND HAVE PROGRESSED FOLLOWING TREATMENT OR HAVE NO SATISFACTORY ALTERNATIVE THERAPY NDA:
Dosage:
PELLETS;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ROZLYTREK
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Hoffmann-La Roche | Phase 2/Phase 3 |
| University of Birmingham | Phase 2/Phase 3 |
| Cancer Research UK | Phase 2/Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for ROZLYTREK
US Patents and Regulatory Information for ROZLYTREK
ROZLYTREK is protected by nineteen US patents and six FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ROZLYTREK is ⤷ Try a Trial.
This potential generic entry date is based on TREATMENT OF PEDIATRIC PATIENTS OLDER THAN 1 MONTH UP TO 12 YEARS OF AGE WITH SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION, AS DETECTED BY AN FDA-APPROVED TEST WITHOUT A KNOWN ACQUIRED RESISTANCE MUTATION, ARE METASTATIC OR WHERE SURGICAL RESECTION IS LIKELY TO RESULT IN SEVERE MORBIDITY, AND HAVE PROGRESSED FOLLOWING TREATMENT OR HAVE NO SATISFACTORY ALTERNATIVE THERAPY.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ROZLYTREK
Molecules for administration to ROS1 mutant cancer cells
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Molecules for administration to ROS1 mutant cancer cells
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ROS1-POSITIVE NON-SMALL CELL LUNG CANCER
Pharmaceutical compositions and dosage forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods for treating neuroblastoma
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NEUROBLASTOMAS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Crystalline form of N-[5-(3,5-difluoro-benzyl)-1H-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2- -(tetrahydro-pyran-4-ylamino)-benzamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF COLORECTAL CANCER THAT HAS A NEUROTROPHIC TYROSINE RECEPTOR KINASE(NTRK) GENE FUSION
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ROS1-POSITIVE NON-SMALL CELL LUNG CANCER
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Pharmaceutical compositions and dosage forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ROS1-POSITIVE NON-SMALL CELL LUNG CANCER
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Process for the preparation of N-[5-(3,5-difluoro-benzyl)-1H-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2- -(tetrahydro-pyran-4-ylamino)-benzamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ROS1-POSITIVE NON-SMALL CELL LUNG CANCER
Substituted indazole derivatives active as kinase inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Treatment of diseases through administration of N-[5-(3,5-difluoro-benzyl)-1H-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2- -(tetrahydro-pyran-4-ylamino)-benzamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION
Treatment of diseases through administration of N-[5-(3,5-difluoro-benzyl)-1H-indazol-3-yl]-4-(4-methyl-piperazin-1-yl)-2- -(tetrahydro-pyran-4-ylamino)-benzamide
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF ROS1-POSITIVE NON-SMALL CELL LUNG CANCER
FDA Regulatory Exclusivity protecting ROZLYTREK
INDICATED FOR THE TREATMENT OF ADULT PATIENTS WITH METASTATIC NON-SMALL CELL LUNG CANCER (NSCLC) WHOSE TUMORS ARE ROS1-POSITIVE
Exclusivity Expiration: ⤷ Try a Trial
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
INDICATED FOR THE TREATMENT OF ADULT AND PEDIATRIC PATIENTS 12 YEARS OF AGE AND OLDER WITH SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION WITHOUT A KNOWN ACQUIRED RESISTANCE MUTATION, ARE METASTATIC OR WHERE SURGICAL RESECTION IS LIKELY TO RESULT IN SEVERE MORBIDITY, AND HAVE EITHER PROGRESSED FOLLOWING TREATMENT OR HAVE NO SATISFACTORY ALTERNATIVE THERAPY
Exclusivity Expiration: ⤷ Try a Trial
NEW PATIENT POPULATION
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF PEDIATRIC PATIENTS OLDER THAN 1 MONTH UP TO 12 YEARS OF AGE WITH SOLID TUMORS THAT HAVE A NEUROTROPHIC TYROSINE RECEPTOR KINASE (NTRK) GENE FUSION, AS DETECTED BY AN FDA-APPROVED TEST WITHOUT A KNOWN ACQUIRED RESISTANCE MUTATION, ARE METASTATIC OR WHERE SURGICAL RESECTION IS LIKELY TO RESULT IN SEVERE MORBIDITY, AND HAVE PROGRESSED FOLLOWING TREATMENT OR HAVE NO SATISFACTORY ALTERNATIVE THERAPY
Exclusivity Expiration: ⤷ Try a Trial
NEW PRODUCT
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genentech Inc | ROZLYTREK | entrectinib | CAPSULE;ORAL | 212725-002 | Aug 15, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Genentech Inc | ROZLYTREK | entrectinib | PELLETS;ORAL | 218550-001 | Oct 20, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Genentech Inc | ROZLYTREK | entrectinib | CAPSULE;ORAL | 212725-002 | Aug 15, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Genentech Inc | ROZLYTREK | entrectinib | PELLETS;ORAL | 218550-001 | Oct 20, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Genentech Inc | ROZLYTREK | entrectinib | CAPSULE;ORAL | 212725-002 | Aug 15, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ROZLYTREK
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Roche Registration GmbH | Rozlytrek | entrectinib | EMEA/H/C/004936 Rozlytrek as monotherapy is indicated for the treatment of adult and paediatric patients 12 years of age and older with solid tumours expressing a neurotrophic tyrosine receptor kinase (NTRK) gene fusion,who have a disease that is locally advanced, metastatic or where surgical resection is likely to result in severe morbidity, andwho have not received a prior NTRK inhibitorwho have no satisfactory treatment options.Rozlytrek as monotherapy is indicated for the treatment of adult patients with ROS1 positive, advanced non small cell lung cancer (NSCLC) not previously treated with ROS1 inhibitors. |
Authorised | no | no | no | 2020-07-31 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ROZLYTREK
When does loss-of-exclusivity occur for ROZLYTREK?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 18302170
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2020000793
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 69339
Estimated Expiration: ⤷ Try a Trial
China
Patent: 0913842
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 54952
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 1759
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 03083
Estimated Expiration: ⤷ Try a Trial
Patent: 20527575
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 200031115
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 85074
Estimated Expiration: ⤷ Try a Trial
Patent: 1907924
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ROZLYTREK around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Singapore | 183049 | SUBSTITUTED INDAZOLE DERIVATIVES ACTIVE AS KINASE INHIBITORS | ⤷ Try a Trial |
| European Patent Office | 3107541 | COMPOSÉS POUR TRAITER DES PATIENTS PRÉSENTANT DES CELLULES CANCÉREUSES MUTANTES ROS1 (COMPOUNDS FOR TREATING PATIENTS WITH ROS1 MUTANT CANCER CELLS) | ⤷ Try a Trial |
| Eurasian Patent Organization | 201070167 | ЗАМЕЩЕННЫЕ ПРОИЗВОДНЫЕ ИНДАЗОЛА, АКТИВНЫЕ КАК ИНГИБИТОРЫ КИНАЗЫ | ⤷ Try a Trial |
| South Korea | 101636312 | ⤷ Try a Trial | |
| Poland | 3290414 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ROZLYTREK
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3107541 | 2021C/522 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ENTRECTINIB IN ALLE VORMEN ZOALS BESCHERMD DOOR HET BASISOCTROOI; AUTHORISATION NUMBER AND DATE: EU/1/20/1460 20200803 |
| 2176231 | 301090 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ENTRECTINIB, DAN WEL TAUTOMEREN, OF FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; REGISTRATION NO/DATE: EU/1/20/1460 20200803 |
| 2176231 | C202030070 | Spain | ⤷ Try a Trial | PRODUCT NAME: ENTRECTINIB O SUS ISOMEROS, TAUTOMEROS, O SALES FARMACEUTICAMENTE ACEPTABLES.; NATIONAL AUTHORISATION NUMBER: EU/1/20/1460; DATE OF AUTHORISATION: 20200731; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1460; DATE OF FIRST AUTHORISATION IN EEA: 20200731 |
| 3107541 | 301111 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ENTRECTINIB OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; REGISTRATION NO/DATE: EU/1/20/1460 20200803 |
| 2176231 | 20C1064 | France | ⤷ Try a Trial | PRODUCT NAME: ENTRECTINIB OU SES TAUTOMERES OU SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/20/1460 20200803 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


