RENAGEL Drug Patent Profile
✉ Email this page to a colleague
When do Renagel patents expire, and what generic alternatives are available?
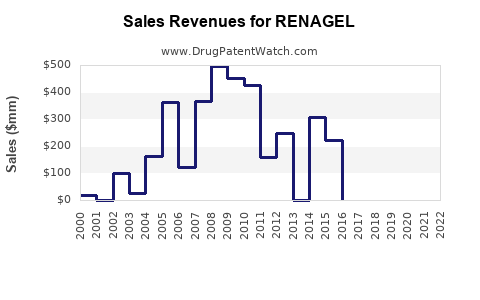
Renagel is a drug marketed by Genzyme and is included in two NDAs.
The generic ingredient in RENAGEL is sevelamer hydrochloride. There are thirty-two drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the sevelamer hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Renagel
A generic version of RENAGEL was approved as sevelamer hydrochloride by GLENMARK PHARMS LTD on February 8th, 2019.
Summary for RENAGEL
| US Patents: | 0 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 50 |
| Clinical Trials: | 19 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for RENAGEL |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for RENAGEL |
| What excipients (inactive ingredients) are in RENAGEL? | RENAGEL excipients list |
| DailyMed Link: | RENAGEL at DailyMed |

Recent Clinical Trials for RENAGEL
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Medical University of Lodz | Phase 4 |
| Shire | Phase 4 |
| Medical Universtity of Lodz | Phase 4 |
Anatomical Therapeutic Chemical (ATC) Classes for RENAGEL
Paragraph IV (Patent) Challenges for RENAGEL
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| RENAGEL | Tablets | sevelamer hydrochloride | 400 mg and 800 mg | 021179 | 1 | 2008-05-22 |
US Patents and Regulatory Information for RENAGEL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genzyme | RENAGEL | sevelamer hydrochloride | CAPSULE;ORAL | 020926-001 | Oct 30, 1998 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Genzyme | RENAGEL | sevelamer hydrochloride | TABLET;ORAL | 021179-001 | Jul 12, 2000 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Genzyme | RENAGEL | sevelamer hydrochloride | TABLET;ORAL | 021179-002 | Jul 12, 2000 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for RENAGEL
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Genzyme | RENAGEL | sevelamer hydrochloride | CAPSULE;ORAL | 020926-001 | Oct 30, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| Genzyme | RENAGEL | sevelamer hydrochloride | TABLET;ORAL | 021179-001 | Jul 12, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Genzyme | RENAGEL | sevelamer hydrochloride | TABLET;ORAL | 021179-001 | Jul 12, 2000 | ⤷ Try a Trial | ⤷ Try a Trial |
| Genzyme | RENAGEL | sevelamer hydrochloride | CAPSULE;ORAL | 020926-001 | Oct 30, 1998 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for RENAGEL
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Genzyme Europe BV | Tasermity | sevelamer hydrochloride | EMEA/H/C/003968 Tasermity is indicated for the control of hyperphosphataemia in adult patients receiving haemodialysis or peritoneal dialysis. Sevelamer hydrochloride should be used within the context of a multiple therapeutic approach, which could include calcium supplements, 1,25 dihydroxy Vitamin D3 or one of its analogues to control the development of renal bone disease., |
Withdrawn | no | no | no | 2015-02-25 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for RENAGEL
See the table below for patents covering RENAGEL around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Australia | 1088701 | ⤷ Try a Trial | |
| Portugal | 716606 | ⤷ Try a Trial | |
| Austria | 286386 | ⤷ Try a Trial | |
| Denmark | 1133989 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for RENAGEL
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0716606 | SPC/GB02/011 200210 | United Kingdom | ⤷ Try a Trial | |
| 0716606 | C00716606/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: SEVELAMER; REGISTRATION NUMBER/DATE: SWISSMEDIC 56297 10.02.2004 |
| 0716606 | 6/2002 | Austria | ⤷ Try a Trial | PRODUCT NAME: SEVELAMER SOWIE DESSEN PHARMAZEUTISCH VERTRAEGLICHEN SALZE; REGISTRATION NO/DATE: EU/1/99/123/001 - EU/1/99/123/004 20000128 |
| 0716606 | CA 2009 00048 | Denmark | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


