QTERNMET XR Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Qternmet Xr, and when can generic versions of Qternmet Xr launch?
Qternmet Xr is a drug marketed by Astrazeneca Ab and is included in one NDA. There are six patents protecting this drug.
This drug has three hundred and forty-three patent family members in forty-eight countries.
The generic ingredient in QTERNMET XR is dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride. There are twenty-six drug master file entries for this compound. Additional details are available on the dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Qternmet Xr
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 12, 2030. This may change due to patent challenges or generic licensing.
There have been sixteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for QTERNMET XR
| International Patents: | 343 |
| US Patents: | 6 |
| Applicants: | 1 |
| NDAs: | 1 |
| Formulation / Manufacturing: | see details |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for QTERNMET XR |
| DailyMed Link: | QTERNMET XR at DailyMed |
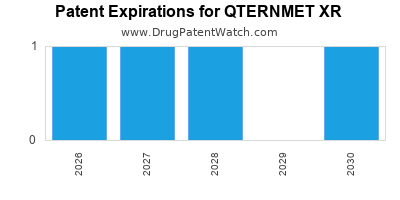
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for QTERNMET XR
Generic Entry Date for QTERNMET XR*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET, EXTENDED RELEASE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Anatomical Therapeutic Chemical (ATC) Classes for QTERNMET XR
US Patents and Regulatory Information for QTERNMET XR
QTERNMET XR is protected by six US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of QTERNMET XR is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting QTERNMET XR
C-aryl glucoside SGLT2 inhibitors and method
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TYPE 2 DIABETES MELLITUS
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TYPE 2 DIABETES MELLITUS
Coated tablet formulation and method
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Bilayer tablet formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-001 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-003 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-004 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-002 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for QTERNMET XR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-001 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-004 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-002 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-001 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for QTERNMET XR
When does loss-of-exclusivity occur for QTERNMET XR?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10319343
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2012011726
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 80939
Estimated Expiration: ⤷ Try a Trial
Patent: 87757
Estimated Expiration: ⤷ Try a Trial
China
Patent: 2711739
Estimated Expiration: ⤷ Try a Trial
Patent: 5193761
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0181347
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 98758
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 98758
Estimated Expiration: ⤷ Try a Trial
Patent: 15124
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 40486
Estimated Expiration: ⤷ Try a Trial
Patent: 000009
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 75522
Estimated Expiration: ⤷ Try a Trial
Patent: 67299
Estimated Expiration: ⤷ Try a Trial
Patent: 22862
Estimated Expiration: ⤷ Try a Trial
Patent: 13510873
Estimated Expiration: ⤷ Try a Trial
Patent: 15110630
Estimated Expiration: ⤷ Try a Trial
Patent: 17081943
Estimated Expiration: ⤷ Try a Trial
Patent: 18172418
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 498758
Estimated Expiration: ⤷ Try a Trial
Patent: 2020003
Estimated Expiration: ⤷ Try a Trial
Patent: 98758
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 5777
Estimated Expiration: ⤷ Try a Trial
Patent: 12005416
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 20009
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 98758
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 98758
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 83920
Estimated Expiration: ⤷ Try a Trial
Patent: 12757
Estimated Expiration: ⤷ Try a Trial
Patent: 12123947
Estimated Expiration: ⤷ Try a Trial
Patent: 16112599
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 756
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 98758
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 89107
Estimated Expiration: ⤷ Try a Trial
Patent: 56888
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering QTERNMET XR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Slovenia | 2139494 | ⤷ Try a Trial | |
| Singapore | 172741 | CRYSTALLINE SOLVATES AND COMPLEXES OF (1S) -1, 5-ANHYDRO-1-C- (3- ( (PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES | ⤷ Try a Trial |
| European Patent Office | 2272825 | Acide amino-hydroxy-adamantane-carboxylique protégé et procédé pour sa préparation (Protected amino hydroxy adamantane carboxylic acid and process for its preparation) | ⤷ Try a Trial |
| Canada | 2653344 | SOLVATES CRISTALLINS ET COMPLEXES DE DERIVES DE (IS)-1,5-ANHYDRO-L-C-{3-[(PHENYL)METHYL]PHENYL}-D-GLUCITOL AVEC DES ACIDES AMINES EN TANT QU'INHIBITEURS DE SGLT2 POUR LE TRAITEMENT DU DIABETE (CRYSTALLINE SOLVATES AND COMPLEXES OF (IS) -1, 5-ANHYDRO-1-C- (3- ( (PHENYL) METHYL) PHENYL) -D-GLUCITOL DERIVATIVES WITH AMINO ACIDS ASSGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for QTERNMET XR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261586 | 2012C/016 | Belgium | ⤷ Try a Trial | PRODUCT NAME: UNE COMBINAISON DE PRODUITS DE SAXAGLIPTINE ET DE METFORMINE AINSI QUE TOUT SELS PHARMACEUTIQUEMENT ACCEPTABLES, DONT LES SELS DE CHLORHYDRATE DE SAXAGLIPTINE ET DE METFORMINE; AUTHORISATION NUMBER AND DATE: EU/1/11/731/001 20111128 |
| 1506211 | C20140021 00131 | Estonia | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOSIIN/METFORMIIN;REG NO/DATE: K(2014)308 (LOPLIK) 21.01.2014 |
| 1506211 | 92496 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE DAPAGLIFLOZINE OU D UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI ET DE METFORMINE OU D UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELLE-CI,TELLE QUE PROTEGEE PAR LE BREVET DE BASE EP1506211 B1 |
| 1506211 | C300677 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN DAPAGLIFLOZINE OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN EN METFORMINE OF EEN FARMACEITISCH AANVAARDBAAR ZOUT DAARVAN, ZOALS BESCHERMD DOOR HET BASISOCTROOI EP 1506211B1; REGISTRATION NO/DATE: EU/1/13/900 20140121 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


