QTERN Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Qtern, and when can generic versions of Qtern launch?
Qtern is a drug marketed by Astrazeneca Ab and is included in two NDAs. There are eight patents protecting this drug and two Paragraph IV challenges.
This drug has three hundred and ten patent family members in forty-eight countries.
The generic ingredient in QTERN is dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride. There are twenty-six drug master file entries for this compound. Additional details are available on the dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Qtern
Qtern was eligible for patent challenges on January 8, 2018.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 16, 2029. This may change due to patent challenges or generic licensing.
There have been sixteen patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for QTERN
| International Patents: | 310 |
| US Patents: | 7 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 1 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for QTERN |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for QTERN |
| What excipients (inactive ingredients) are in QTERN? | QTERN excipients list |
| DailyMed Link: | QTERN at DailyMed |
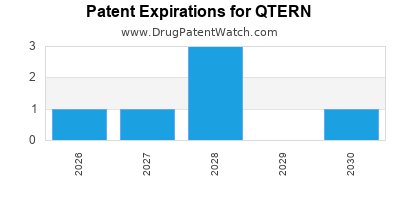
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for QTERN
Generic Entry Date for QTERN*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Pharmacology for QTERN
Anatomical Therapeutic Chemical (ATC) Classes for QTERN
Paragraph IV (Patent) Challenges for QTERN
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| QTERN | Tablets | dapagliflozin; saxagliptin hydrochloride | 5 mg/5 mg | 209091 | 1 | 2020-07-29 |
| QTERN | Tablets | dapagliflozin; saxagliptin hydrochloride | 10 mg/5 mg | 209091 | 5 | 2018-01-08 |
US Patents and Regulatory Information for QTERN
QTERN is protected by eleven US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of QTERN is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting QTERN
C-aryl glucoside SGLT2 inhibitors and method
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TYPE 2 DIABETES MELLITUS
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TYPE 2 DIABETES MELLITUS
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD FOR TREATING TYPE 2 DIABETES MELLITUS (T2DM) IN PATIENTS WHO ARE ALREADY TREATED WITH DAPAGLIFLOZIN AND SAXAGLIPTIN
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD FOR TREATING TYPE 2 DIABETES MELLITUS (T2DM) IN PATIENTS WHO HAVE INADEQUATE CONTROL WITH DAPAGLIFLOZIN
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF TYPE 2 DIABETES MELLITUS
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD FOR TREATING TYPE 2 DIABETES MELLITUS (T2DM) IN PATIENTS WHO HAVE INADEQUATE CONTROL WITH DAPAGLIFLOZIN
Crystal structures of SGLT2 inhibitors and processes for preparing same
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD FOR TREATING TYPE 2 DIABETES MELLITUS (T2DM) IN PATIENTS WHO ARE ALREADY TREATED WITH DAPAGLIFLOZIN AND SAXAGLIPTIN
Coated tablet formulation and method
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Pharmaceutical formulations containing an SGLT2 inhibitor
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-004 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-003 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-002 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-003 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERNMET XR | dapagliflozin; metformin hydrochloride; saxagliptin hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 210874-002 | May 2, 2019 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for QTERN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-002 | May 2, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Astrazeneca Ab | QTERN | dapagliflozin; saxagliptin hydrochloride | TABLET;ORAL | 209091-001 | Feb 27, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for QTERN
When does loss-of-exclusivity occur for QTERN?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 1730
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 07265246
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0713544
Estimated Expiration: ⤷ Try a Trial
Patent: 2017015106
Estimated Expiration: ⤷ Try a Trial
Patent: 2017021516
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 53344
Estimated Expiration: ⤷ Try a Trial
Patent: 24318
Estimated Expiration: ⤷ Try a Trial
Patent: 85797
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 07001915
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1479287
Estimated Expiration: ⤷ Try a Trial
Patent: 3145773
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 60299
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0141007
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 15738
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 69374
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 8229
Estimated Expiration: ⤷ Try a Trial
Patent: 0428
Estimated Expiration: ⤷ Try a Trial
Patent: 8259
Estimated Expiration: ⤷ Try a Trial
Patent: 5999
Estimated Expiration: ⤷ Try a Trial
Patent: 0900066
Estimated Expiration: ⤷ Try a Trial
Patent: 1171333
Estimated Expiration: ⤷ Try a Trial
Patent: 1490902
Estimated Expiration: ⤷ Try a Trial
Patent: 1791254
Estimated Expiration: ⤷ Try a Trial
Patent: 2091391
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 69374
Estimated Expiration: ⤷ Try a Trial
Patent: 57918
Estimated Expiration: ⤷ Try a Trial
Patent: 45466
Estimated Expiration: ⤷ Try a Trial
Patent: 63807
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 27359
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 5882
Estimated Expiration: ⤷ Try a Trial
Patent: 4180
Estimated Expiration: ⤷ Try a Trial
Patent: 4181
Estimated Expiration: ⤷ Try a Trial
Patent: 4182
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 13889
Estimated Expiration: ⤷ Try a Trial
Patent: 66651
Estimated Expiration: ⤷ Try a Trial
Patent: 37187
Estimated Expiration: ⤷ Try a Trial
Patent: 09545525
Estimated Expiration: ⤷ Try a Trial
Patent: 13209394
Estimated Expiration: ⤷ Try a Trial
Patent: 15071636
Estimated Expiration: ⤷ Try a Trial
Patent: 16172758
Estimated Expiration: ⤷ Try a Trial
Patent: 17222681
Estimated Expiration: ⤷ Try a Trial
Patent: 19059779
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 8566
Estimated Expiration: ⤷ Try a Trial
Patent: 3930
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 9143
Estimated Expiration: ⤷ Try a Trial
Patent: 7155
Estimated Expiration: ⤷ Try a Trial
Patent: 08015377
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 4346
Estimated Expiration: ⤷ Try a Trial
Patent: 9190
Estimated Expiration: ⤷ Try a Trial
Patent: 9195
Estimated Expiration: ⤷ Try a Trial
Patent: 9202
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 6828
Estimated Expiration: ⤷ Try a Trial
Patent: 085169
Estimated Expiration: ⤷ Try a Trial
Patent: 221233
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 080349
Estimated Expiration: ⤷ Try a Trial
Patent: 120776
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 012500168
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 69374
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 69374
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 638
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 2741
Estimated Expiration: ⤷ Try a Trial
Patent: 201402181S
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 69374
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0810475
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1493102
Estimated Expiration: ⤷ Try a Trial
Patent: 090023643
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 21665
Estimated Expiration: ⤷ Try a Trial
Patent: 59862
Estimated Expiration: ⤷ Try a Trial
Patent: 69130
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 21245
Estimated Expiration: ⤷ Try a Trial
Patent: 66876
Estimated Expiration: ⤷ Try a Trial
Patent: 19528
Estimated Expiration: ⤷ Try a Trial
Patent: 0811127
Estimated Expiration: ⤷ Try a Trial
Patent: 1406743
Estimated Expiration: ⤷ Try a Trial
Patent: 1509927
Estimated Expiration: ⤷ Try a Trial
Patent: 1546054
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 765
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering QTERN around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Hungary | S1200031 | ⤷ Try a Trial | |
| New Zealand | 589202 | CRYSTALLINE SOLVATES AND COMPLEXES OF (1S)-1,5-ANHYDRO-1-C-(3-((PHENYL) METHYL) PHENYL)-D-GLUCITOL DERIVATIVES WITH AMINO ACIDS AS SGLT2 INHIBITORS FOR THE TREATMENT OF DIABETES | ⤷ Try a Trial |
| Czech Republic | 304355 | 2-Azabicyklo[3.1.0.]hexan-3-karbonitrilová sloučenina, farmaceutický prostředek a použití (2-Azabicyclo [3.1.0]hexane-3-carbonitrile compound, pharmaceutical composition and use thereof) | ⤷ Try a Trial |
| Japan | 2019059779 | 糖尿病の治療用SGLT2阻害剤としてのアミノ酸を有する(1S)−1,5−アンヒドロ−1−C−(3−((フェニル)メチル)フェニル)−D−グルシトール誘導体の結晶性溶媒和物および複合体 (CRYSTALLINE SOLVATES AND COMPLEXES OF (1S)-1,5-ANHYDRO-1-C-(3-((PHENYL)METHYL)PHENYL)-D-GLUCITOL DERIVATIVES HAVING AMINO ACIDS AS SGLT2 INHIBITORS FOR TREATING DIABETES) | ⤷ Try a Trial |
| Eurasian Patent Organization | 201171333 | КРИСТАЛЛИЧЕСКИЕ СОЛЬВАТЫ И КОМПЛЕКСЫ ПРОИЗВОДНЫХ (1S)-1,5-АНГИДРО-1-С-(3-((ФЕНИЛ)МЕТИЛ)ФЕНИЛ)-D-ГЛЮЦИТОЛА С АМИНОКИСЛОТАМИ В КАЧЕСТВЕ ИНГИБИТОРОВ SGLT2 ДЛЯ ЛЕЧЕНИЯ ДИАБЕТА | ⤷ Try a Trial |
| Russian Federation | 2489151 | ФАРМАЦЕВТИЧЕСКАЯ КОМБИНАЦИЯ, СОДЕРЖАЩАЯ ИНГИБИТОР SGLT2 (PHARMACEUTICAL COMBINATION CONTAINING SGLT2 INHIBITOR) | ⤷ Try a Trial |
| Serbia | 55174 | FORMULACIJA I METOD OBLOŽENE TABLETE (COATED TABLET FORMULATION AND METHOD) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for QTERN
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1261586 | C300524 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN SAXAGLIPTINE EN METFORMINE, DESGEWENST IN DE VORM VAN FARMACEUTISCH AANVAARDBARE AFGELEIDEN DAARVAN; NAT. REGISTRATION NO/DATE: EU/1/11/731/001-012 20111124; FIRST REGISTRATION: |
| 1506211 | 122014000071 | Germany | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON DAPAGLIFLOZIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON, UND METFORMIN ODER EINEM PHARMAZEUTISCH VERTRAEGLICHEN SALZ DAVON, GESCHUETZT DURCH DAS GRUNDPATENT EP 1 506 211; REGISTRATION NO/DATE: EU/1/13/900 20140116 |
| 1506211 | 13C0022 | France | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZINE ET SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; REGISTRATION NO/DATE: EU/1/12/795/001 20121112 |
| 1261586 | 15/2012 | Austria | ⤷ Try a Trial | PRODUCT NAME: KOMBINATIONSPRODUKT VON SAXAGLIPTIN UND METFORMIN UND PHARMAZEUTISCH VERTRAEGLICHE SALZE DAVON, BEINHALTEND DIE HYDROCHLORIDSALZE VON SAXAGLIPTIN UND METFORMIN; REGISTRATION NO/DATE: EU/1/11/731/001-EU/1/11/731/012 20111124 |
| 1261586 | 12C0028 | France | ⤷ Try a Trial | PRODUCT NAME: ASSOCIATION COMPRENANT LA SAXAGLIPTINE OU UN DE SES SELS ET LA METFORMINE OU UN DE SES SELS, Y COMPRIS L'ASSOCIATION CHLORHYDRATE DE SAXAGLIPTINE ET CHLORHYDRATE DE METFORMINE; REGISTRATION NO/DATE: EU/1/11/731/001 20111124 |
| 1506211 | PA 2013 008, C 1506211 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZINUM; REGISTRATION NO/DATE: EU/1/12/795/001 - EU/1/12/795/010 20121112 |
| 1506211 | PA2013008,C1506211 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DAPAGLIFLOZINUM; REGISTRATION NO/DATE: EU/1/12/795/001 - EU/1/12/795/010 20121112 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


