PICATO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Picato, and what generic alternatives are available?
Picato is a drug marketed by Leo Labs and is included in one NDA. There are twelve patents protecting this drug and one Paragraph IV challenge.
This drug has thirty-five patent family members in twenty-one countries.
The generic ingredient in PICATO is ingenol mebutate. There are three drug master file entries for this compound. Additional details are available on the ingenol mebutate profile page.
DrugPatentWatch® Generic Entry Outlook for Picato
Picato was eligible for patent challenges on January 23, 2016.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There is one tentative approval for the generic drug (ingenol mebutate), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for PICATO
| International Patents: | 35 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 31 |
| Clinical Trials: | 19 |
| Patent Applications: | 542 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for PICATO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for PICATO |
| What excipients (inactive ingredients) are in PICATO? | PICATO excipients list |
| DailyMed Link: | PICATO at DailyMed |

Recent Clinical Trials for PICATO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Instituto Nacional de Cancer, Brazil | Phase 1/Phase 2 |
| LEO Pharma | Early Phase 1 |
| Center for Clinical Studies, Texas | Early Phase 1 |
Anatomical Therapeutic Chemical (ATC) Classes for PICATO
Paragraph IV (Patent) Challenges for PICATO
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PICATO | Gel | ingenol mebutate | 0.015% | 202833 | 2 | 2016-01-27 |
US Patents and Regulatory Information for PICATO
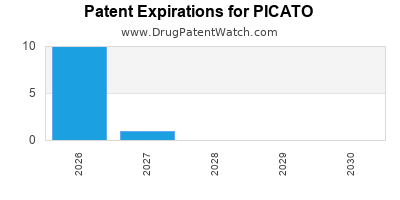
PICATO is protected by twelve US patents.
Patents protecting PICATO
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF INGENOL MEBUTATE TO TREAT ACTINIC KERATOSIS
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF INGENOL MEBUTATE TO TREAT ACTINIC KERATOSIS
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF INGENOL MEBUTATE TO TREAT ACTINIC KERATOSIS
Method of topically treating actinic keratosis with ingenol mebutate cycle therapy
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TOPICAL TREATMENT OF ACTINIC KERATOSIS OF THE FACE OR SCALP USING MORE THAN ONE TREATMENT COURSE OF INGENOL MEBUTATE
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF INGENOL MEBUTATE TO TREAT ACTINIC KERATOSIS
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Therapeutic compositions
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: USE OF INGENOL MEBUTATE TO TREAT ACTINIC KERATOSIS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-001 | Jan 23, 2012 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-002 | Jan 23, 2012 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-001 | Jan 23, 2012 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-001 | Jan 23, 2012 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PICATO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-001 | Jan 23, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-002 | Jan 23, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-001 | Jan 23, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Leo Labs | PICATO | ingenol mebutate | GEL;TOPICAL | 202833-002 | Jan 23, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for PICATO
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| LEO Laboratories Ltd. | Picato | ingenol mebutate | EMEA/H/C/002275 Picato is indicated for the cutaneous treatment of non‑hyperkeratotic, non‑hypertrophic actinic keratosis in adults. |
Withdrawn | no | no | no | 2012-11-15 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for PICATO
When does loss-of-exclusivity occur for PICATO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06325244
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0619919
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 34073
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 15292
Estimated Expiration: ⤷ Try a Trial
Patent: 14030
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 88877
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 8545
Estimated Expiration: ⤷ Try a Trial
Patent: 4152
Estimated Expiration: ⤷ Try a Trial
Patent: 0870063
Estimated Expiration: ⤷ Try a Trial
Patent: 1201452
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 88877
Estimated Expiration: ⤷ Try a Trial
Patent: 99571
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 20998
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 2221
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 84733
Estimated Expiration: ⤷ Try a Trial
Patent: 09826
Estimated Expiration: ⤷ Try a Trial
Patent: 09519314
Estimated Expiration: ⤷ Try a Trial
Patent: 13049715
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 08007685
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 9197
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 4271
Estimated Expiration: ⤷ Try a Trial
Patent: 083150
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 88877
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 88877
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 88877
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0805187
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1451993
Estimated Expiration: ⤷ Try a Trial
Patent: 1593579
Estimated Expiration: ⤷ Try a Trial
Patent: 080092373
Estimated Expiration: ⤷ Try a Trial
Patent: 140088617
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 61315
Estimated Expiration: ⤷ Try a Trial
United Kingdom
Patent: 25680
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering PICATO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 101451993 | ⤷ Try a Trial | |
| Germany | 69839586 | ⤷ Try a Trial | |
| European Patent Office | 2399571 | Compositions thérapeutiques comprenant de l'ingénol-2-angelate (Therapeutic compositions comprising ingenol-3-angelate) | ⤷ Try a Trial |
| Eurasian Patent Organization | 201201452 | ТЕРАПЕВТИЧЕСКИЕ КОМПОЗИЦИИ | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for PICATO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1988877 | 300682 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: INGENOLMEBUTAAT OF EEN DERIVAAT (ZOUT OF ESTER) DAARVAN; REGISTRATION NO/DATE: EU/1/12/796 20121119 |
| 1015413 | C300592 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: INGENAN OF EEN DERIVAAT (ZOUT OF ESTER) DAARVAN; REGISTRATION NO/DATE: EU/1/12/796/001-002 20121115 |
| 1988877 | 122014000075 | Germany | ⤷ Try a Trial | PRODUCT NAME: INGENOLMEBUTAT ODER EIN DERIVAT (SALZ ODER ESTER) DAVON; REGISTRATION NO/DATE: EU/1/12/796 20121115 |
| 1988877 | C300682 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: INGENOLMEBUTAAT OF EEN DERIVAAT (ZOUT OF ESTER) DAARVAN; REGISTRATION NO/DATE: EU/1/12/796 20121119 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


