PHEXXI Drug Patent Profile
✉ Email this page to a colleague
When do Phexxi patents expire, and when can generic versions of Phexxi launch?
Phexxi is a drug marketed by Evofem Inc and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-five patent family members in twenty-three countries.
The generic ingredient in PHEXXI is citric acid; lactic acid; potassium bitartrate. There are five drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the citric acid; lactic acid; potassium bitartrate profile page.
DrugPatentWatch® Generic Entry Outlook for Phexxi
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for PHEXXI
| International Patents: | 65 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 3 |
| Formulation / Manufacturing: | see details |
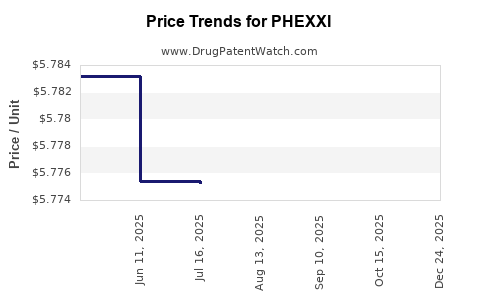
| Drug Prices: | Drug price information for PHEXXI |
| What excipients (inactive ingredients) are in PHEXXI? | PHEXXI excipients list |
| DailyMed Link: | PHEXXI at DailyMed |
Recent Clinical Trials for PHEXXI
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| University of Hawaii Foundation | Early Phase 1 |
| Queen's Medical Center | Early Phase 1 |
| Parexel | Phase 3 |
Pharmacology for PHEXXI
| Drug Class | Anti-coagulant Calculi Dissolution Agent |
| Mechanism of Action | Acidifying Activity Calcium Chelating Activity |
| Physiological Effect | Decreased Coagulation Factor Activity |
Anatomical Therapeutic Chemical (ATC) Classes for PHEXXI
Paragraph IV (Patent) Challenges for PHEXXI
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| PHEXXI | Vaginal Gel | citric acid; lactic acid; potassium bitartrate | 1.8%/1%/0.4% | 208352 | 1 | 2023-02-28 |
US Patents and Regulatory Information for PHEXXI
PHEXXI is protected by four US patents.
Patents protecting PHEXXI
Compositions and methods for enhancing the efficacy of contraceptive microbicides
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY
Compositions and methods for inhibiting inflammation and diseases using an alginic acid-based antimicrobial compound
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: PREVENTION OF PREGNANCY
Compositions and methods for enhancing the efficacy of contraceptive microbicides
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for trapping and inactivating pathogenic microbes and spermatozoa
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for PHEXXI
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Evofem Inc | PHEXXI | citric acid; lactic acid; potassium bitartrate | GEL;VAGINAL | 208352-001 | May 22, 2020 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for PHEXXI
See the table below for patents covering PHEXXI around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20150018636 | COMPOSITIONS AND METHODS FOR ENHANCING THE EFFICACY OF CONTRACEPTIVE MICROBICIDES | ⤷ Try a Trial |
| European Patent Office | 2861215 | COMPOSITIONS ET MÉTHODES D'AMÉLIORATION DE L'EFFICACITÉ DE MICROBICIDES CONTRACEPTIFS (COMPOSITIONS AND METHODS FOR ENHANCING THE EFFICACY OF CONTRACEPTIVE MICROBICIDES) | ⤷ Try a Trial |
| China | 114452298 | 使用基于藻酸的抗微生物化合物抑制炎症和疾病的组合物和方法 (Compositions and methods for inhibiting inflammation and diseases using alginic acid-based antimicrobial compounds) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


