OZEMPIC Drug Patent Profile
✉ Email this page to a colleague
When do Ozempic patents expire, and what generic alternatives are available?
Ozempic is a drug marketed by Novo and is included in one NDA. There are twenty-one patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and twenty-six patent family members in twenty-eight countries.
The generic ingredient in OZEMPIC is semaglutide. Two suppliers are listed for this compound. Additional details are available on the semaglutide profile page.
DrugPatentWatch® Generic Entry Outlook for Ozempic
Ozempic was eligible for patent challenges on December 5, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 5, 2031. This may change due to patent challenges or generic licensing.
There have been twenty-one patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for OZEMPIC
| International Patents: | 226 |
| US Patents: | 21 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 16 |
| Clinical Trials: | 29 |
| Patent Applications: | 938 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for OZEMPIC |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for OZEMPIC |
| What excipients (inactive ingredients) are in OZEMPIC? | OZEMPIC excipients list |
| DailyMed Link: | OZEMPIC at DailyMed |
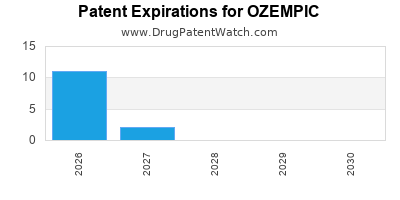
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for OZEMPIC
Generic Entry Date for OZEMPIC*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;SUBCUTANEOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for OZEMPIC
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Heart, Lung, and Blood Institute (NHLBI) | Phase 3 |
| University of Colorado, Denver | Phase 3 |
| Western University, Canada | Phase 4 |
Pharmacology for OZEMPIC
| Drug Class | GLP-1 Receptor Agonist |
| Mechanism of Action | Glucagon-like Peptide-1 (GLP-1) Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for OZEMPIC
Paragraph IV (Patent) Challenges for OZEMPIC
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| OZEMPIC | Injection | semaglutide | 8 mg/3 mL | 209637 | 1 | 2022-12-21 |
| OZEMPIC | Injection | semaglutide | 2 mg/1.5 mL and 4 mg/3 mL | 209637 | 7 | 2021-12-06 |
US Patents and Regulatory Information for OZEMPIC
OZEMPIC is protected by twenty-four US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of OZEMPIC is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting OZEMPIC
Syringe device with a dose limiting mechanism and an additional safety mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of long-acting GLP-1 peptides
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATING TYPE 2 DIABETES COMPRISING ADMINISTERING SEMAGLUTIDE ONCE WEEKLY IN A AMOUNT OF 1.0 MG TO A SUBJECT IN NEED THEREOF
Injection device with an end of dose feedback mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Automatic injection device with a top release mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Syringe device with a dose limiting mechanism and an additional safety mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Automatic injection device with a top release mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Needle mounting system and a method for mounting a needle assembly
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Propylene glycol-containing peptide formulations which are optimal for production and for use in injection devices
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Acylated GLP-1 compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: OZEMPIC IS INDICATED AS AN ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES MELLITUS
Acylated GLP-1 compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: AN ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES MELLITUS
Acylated GLP-1 compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: OZEMPIC IS INDICATED AS AN ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES MELLITUS
Acylated GLP-1 compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: AN ADJUNCT TO DIET AND EXERCISE TO IMPROVE GLYCEMIC CONTROL IN ADULTS WITH TYPE 2 DIABETES MELLITUS
Injection device with torsion spring and rotatable display
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dose mechanism for an injection device for limiting a dose setting corresponding to the amount of medicament left
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Automatic injection device with a top release mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dial-down mechanism for wind-up pen
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Injection device with an end of dose feedback mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Injection device with an end of dose feedback mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Automatic injection device with a top release mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Injection device with torsion spring and rotatable display
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dose mechanism for an injection device for limiting a dose setting corresponding to the amount of medicament left
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Injection device with an end of dose feedback mechanism
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Dial-down mechanism for wind-up pen
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting OZEMPIC
ADDITION OF A 3RD MAINTENANCE DOSE OF SEMAGLUTIDE
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-003 | Mar 28, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-004 | Oct 6, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-004 | Oct 6, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for OZEMPIC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-001 | Dec 5, 2017 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-003 | Mar 28, 2022 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novo | OZEMPIC | semaglutide | SOLUTION;SUBCUTANEOUS | 209637-002 | Apr 9, 2019 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for OZEMPIC
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novo Nordisk A/S | Wegovy | semaglutide | EMEA/H/C/005422 Wegovy is indicated as an adjunct to a reduced-calorie diet and increased physical activity for weight management, including weight loss and weight maintenance, in adults with an initial Body Mass Index (BMI) of- ≥30 kg/m² (obesity), or- ≥27 kg/m² to |
Authorised | no | no | no | 2022-01-06 | |
| Novo Nordisk A/S | Ozempic | semaglutide | EMEA/H/C/004174 Treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise:as monotherapy when metformin is considered inappropriate due to intolerance or contraindications;in addition to other medicinal products for the treatment of diabetes.For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. |
Authorised | no | no | no | 2018-02-08 | |
| Novo Nordisk A/S | Rybelsus | semaglutide | EMEA/H/C/004953 Rybelsus is indicated for the treatment of adults with insufficiently controlled type 2 diabetes mellitus to improve glycaemic control as an adjunct to diet and exerciseas monotherapy when metformin is considered inappropriate due to intolerance or contraindicationsin combination with other medicinal products for the treatment of diabetes.For study results with respect to combinations, effects on glycaemic control and cardiovascular events, and the populations studied, see sections 4.4, 4.5 and 5.1. |
Authorised | no | no | no | 2020-04-03 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for OZEMPIC
When does loss-of-exclusivity occur for OZEMPIC?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 06224536
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 76446
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0607762
Estimated Expiration: ⤷ Try a Trial
Patent: 2019002626
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 01784
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1133082
Estimated Expiration: ⤷ Try a Trial
Patent: 4017062
Estimated Expiration: ⤷ Try a Trial
Patent: 4402989
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 63839
Estimated Expiration: ⤷ Try a Trial
Patent: 22546
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 63839
Estimated Expiration: ⤷ Try a Trial
Patent: 22546
Estimated Expiration: ⤷ Try a Trial
Germany
Patent: 2006015928
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 28194
Estimated Expiration: ⤷ Try a Trial
Patent: 800019
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 4051
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 85037
Estimated Expiration: ⤷ Try a Trial
Patent: 09463
Estimated Expiration: ⤷ Try a Trial
Patent: 08533105
Estimated Expiration: ⤷ Try a Trial
Patent: 10116407
Estimated Expiration: ⤷ Try a Trial
Patent: 13063984
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 07011220
Estimated Expiration: ⤷ Try a Trial
Netherlands
Patent: 0936
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 7946
Estimated Expiration: ⤷ Try a Trial
Patent: 18023
Estimated Expiration: ⤷ Try a Trial
Patent: 075342
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 63839
Estimated Expiration: ⤷ Try a Trial
Patent: 22546
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 63839
Estimated Expiration: ⤷ Try a Trial
Patent: 22546
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 34019
Estimated Expiration: ⤷ Try a Trial
Patent: 07134156
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0707261
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1205272
Estimated Expiration: ⤷ Try a Trial
Patent: 070120089
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 50051
Estimated Expiration: ⤷ Try a Trial
Patent: 57313
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 62392
Estimated Expiration: ⤷ Try a Trial
Patent: 72629
Estimated Expiration: ⤷ Try a Trial
Patent: 0700433
Estimated Expiration: ⤷ Try a Trial
Patent: 0942255
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OZEMPIC around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Austria | E444090 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 02053214 | ⤷ Try a Trial | |
| Germany | 602006010396 | ⤷ Try a Trial | |
| Australia | 2009306387 | Dial-down mechanism for wind-up pen | ⤷ Try a Trial |
| Canada | 2595323 | DISPOSITIF D'INJECTION PRESENTANT UNE EXTREMITE DE MECANISMEDE RETROACTION DE DOSE (AN INJECTION DEVICE WITH AN END OF DOSE FEEDBACK MECHANISM) | ⤷ Try a Trial |
| Russian Federation | 2007134156 | АЦИЛИРОВАННЫЕ GLP-1 СОЕДИНЕНИЯ | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OZEMPIC
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1863839 | C01863839/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: SEMAGLUTIDE; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 66604 02.07.2018 |
| 1863839 | 2018/017 | Ireland | ⤷ Try a Trial | PRODUCT NAME: OZEMPIC-SEMAGLUTIDE; REGISTRATION NO/DATE: EU/1/17/1251 20180208 |
| 1863839 | 661 | Finland | ⤷ Try a Trial | |
| 1863839 | 300936 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SEMAGLUTIDE; REGISTRATION NO/DATE: EU/1/17/1251 20180208 |
| 1863839 | CR 2018 00019 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SEMAGLUTID; REG. NO/DATE: EU/1/17/1251 20180212 |
| 1863839 | 18C1017 | France | ⤷ Try a Trial | PRODUCT NAME: SEMAGLUTIDE; REGISTRATION NO/DATE: EU/1/17/1251 20180212 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


