ONPATTRO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Onpattro, and what generic alternatives are available?
Onpattro is a drug marketed by Alnylam Pharms Inc and is included in one NDA. There are fourteen patents protecting this drug.
This drug has two hundred and forty-four patent family members in thirty-one countries.
The generic ingredient in ONPATTRO is patisiran sodium. One supplier is listed for this compound. Additional details are available on the patisiran sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Onpattro
Onpattro was eligible for patent challenges on August 10, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be November 10, 2030. This may change due to patent challenges or generic licensing.
There have been four patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for ONPATTRO
| International Patents: | 244 |
| US Patents: | 14 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 1 |
| Drug Prices: | Drug price information for ONPATTRO |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ONPATTRO |
| What excipients (inactive ingredients) are in ONPATTRO? | ONPATTRO excipients list |
| DailyMed Link: | ONPATTRO at DailyMed |
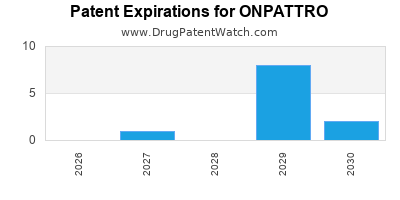

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ONPATTRO
Generic Entry Date for ONPATTRO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SOLUTION;INTRAVENOUS |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ONPATTRO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Austin Neuromuscular Center | Early Phase 1 |
| Alnylam Pharmaceuticals | Early Phase 1 |
Pharmacology for ONPATTRO
| Drug Class | Small Interfering RNA Transthyretin-directed RNA Interaction |
| Physiological Effect | Decreased RNA Integrity Increased Protein Breakdown |
Anatomical Therapeutic Chemical (ATC) Classes for ONPATTRO
US Patents and Regulatory Information for ONPATTRO
ONPATTRO is protected by fourteen US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ONPATTRO is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ONPATTRO
Compositions and methods for inhibiting expression of transthyretin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Methods of treating transthyretin (TTR) mediated amyloidosis
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Lipid formulations for nucleic acid delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lipid formulations for nucleic acid delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lipid formulation
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Compositions and methods for inhibiting expression of transthyretin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Nuclease resistant double-stranded ribonucleic acid
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lipid formulations for nucleic acid delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Lipid containing formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for inhibiting expression of transthyretin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Lipid formulation
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Lipid formulations for nucleic acid delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Compositions and methods for inhibiting expression of transthyretin
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
Lipid formulations for nucleic acid delivery
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS
FDA Regulatory Exclusivity protecting ONPATTRO
INDICATED FOR THE TREATMENT OF THE POLYNEUROPATHY OF HEREDITARY TRANSTHYRETIN-MEDIATED AMYLOIDOSIS IN ADULTS
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for ONPATTRO
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| Alnylam Pharms Inc | ONPATTRO | patisiran sodium | SOLUTION;INTRAVENOUS | 210922-001 | Aug 10, 2018 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for ONPATTRO
When does loss-of-exclusivity occur for ONPATTRO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 10259984
Estimated Expiration: ⤷ Try a Trial
Patent: 17202702
Estimated Expiration: ⤷ Try a Trial
Patent: 19204984
Estimated Expiration: ⤷ Try a Trial
Patent: 21201228
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 64609
Estimated Expiration: ⤷ Try a Trial
Patent: 14827
Estimated Expiration: ⤷ Try a Trial
China
Patent: 2625696
Estimated Expiration: ⤷ Try a Trial
Patent: 4873464
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0181221
Estimated Expiration: ⤷ Try a Trial
Patent: 0211619
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 20641
Estimated Expiration: ⤷ Try a Trial
Patent: 24769
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 40183
Estimated Expiration: ⤷ Try a Trial
Patent: 31076
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 4960
Estimated Expiration: ⤷ Try a Trial
Patent: 8860
Estimated Expiration: ⤷ Try a Trial
Patent: 1190306
Estimated Expiration: ⤷ Try a Trial
Patent: 1690312
Estimated Expiration: ⤷ Try a Trial
Patent: 1791744
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 40183
Estimated Expiration: ⤷ Try a Trial
Patent: 31076
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 12620
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 38796
Estimated Expiration: ⤷ Try a Trial
Patent: 56773
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6876
Estimated Expiration: ⤷ Try a Trial
Patent: 4945
Estimated Expiration: ⤷ Try a Trial
Patent: 4826
Estimated Expiration: ⤷ Try a Trial
Patent: 0077
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 19291
Estimated Expiration: ⤷ Try a Trial
Patent: 32321
Estimated Expiration: ⤷ Try a Trial
Patent: 59719
Estimated Expiration: ⤷ Try a Trial
Patent: 92144
Estimated Expiration: ⤷ Try a Trial
Patent: 12530059
Estimated Expiration: ⤷ Try a Trial
Patent: 15232048
Estimated Expiration: ⤷ Try a Trial
Patent: 17122126
Estimated Expiration: ⤷ Try a Trial
Patent: 18141019
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 40183
Estimated Expiration: ⤷ Try a Trial
Patent: 31076
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 2785
Estimated Expiration: ⤷ Try a Trial
Patent: 7665
Estimated Expiration: ⤷ Try a Trial
Patent: 11013320
Estimated Expiration: ⤷ Try a Trial
Patent: 19010340
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 6958
Estimated Expiration: ⤷ Try a Trial
Patent: 2843
Estimated Expiration: ⤷ Try a Trial
Patent: 2719
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 40183
Estimated Expiration: ⤷ Try a Trial
Patent: 31076
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 40183
Estimated Expiration: ⤷ Try a Trial
Patent: 31076
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 6786
Estimated Expiration: ⤷ Try a Trial
Patent: 201403054S
Estimated Expiration: ⤷ Try a Trial
Patent: 201912450X
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 40183
Estimated Expiration: ⤷ Try a Trial
Patent: 31076
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1766408
Estimated Expiration: ⤷ Try a Trial
Patent: 1987962
Estimated Expiration: ⤷ Try a Trial
Patent: 2066189
Estimated Expiration: ⤷ Try a Trial
Patent: 2205886
Estimated Expiration: ⤷ Try a Trial
Patent: 2374518
Estimated Expiration: ⤷ Try a Trial
Patent: 120081065
Estimated Expiration: ⤷ Try a Trial
Patent: 170091798
Estimated Expiration: ⤷ Try a Trial
Patent: 190065474
Estimated Expiration: ⤷ Try a Trial
Patent: 200006176
Estimated Expiration: ⤷ Try a Trial
Patent: 210008938
Estimated Expiration: ⤷ Try a Trial
Patent: 220038506
Estimated Expiration: ⤷ Try a Trial
Patent: 230098713
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 89168
Estimated Expiration: ⤷ Try a Trial
Patent: 01627
Estimated Expiration: ⤷ Try a Trial
Turkey
Patent: 1811076
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ONPATTRO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Spain | 2380171 | ⤷ Try a Trial | |
| European Patent Office | 2361981 | Médiateurs spécifiques de la séquence d'ARN d'interférence d'ARN (RNA sequence-specific mediators of RNA interference) | ⤷ Try a Trial |
| Denmark | 2348134 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 2005121368 | ⤷ Try a Trial | |
| Japan | 2004526422 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ONPATTRO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2937418 | 1990004-2 | Sweden | ⤷ Try a Trial | PRODUCT NAME: PATISIRAN; REG. NO/DATE: EU/1/18/1320 20180829 |
| 2937418 | 122018000133 | Germany | ⤷ Try a Trial | PRODUCT NAME: PATISIRAN; REGISTRATION NO/DATE: EU/1/18/1320 20180827 |
| 2937418 | 2019/004 | Ireland | ⤷ Try a Trial | PRODUCT NAME: PATISIRAN; REGISTRATION NO/DATE: EU/1/18/1320 20180827 |
| 2937418 | CA 2019 00005 | Denmark | ⤷ Try a Trial | PRODUCT NAME: PATISIRAN... (NAVN FOR LANGT); REG. NO/DATE: EU/1/18/1320 20180829 |
| 2937418 | 132019000000019 | Italy | ⤷ Try a Trial | PRODUCT NAME: PATISIRAN(ONPATTRO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1320, 20180829 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


