OLYSIO Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Olysio, and what generic alternatives are available?
Olysio is a drug marketed by Janssen Prods and is included in one NDA. There are nine patents protecting this drug.
This drug has one hundred and thirty-nine patent family members in forty-three countries.
The generic ingredient in OLYSIO is simeprevir sodium. There is one drug master file entry for this compound. Additional details are available on the simeprevir sodium profile page.
DrugPatentWatch® Generic Entry Outlook for Olysio
Olysio was eligible for patent challenges on November 22, 2017.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be September 5, 2029. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for OLYSIO
| International Patents: | 139 |
| US Patents: | 9 |
| Applicants: | 1 |
| NDAs: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 49 |
| Clinical Trials: | 7 |
| Patent Applications: | 698 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for OLYSIO |
| DailyMed Link: | OLYSIO at DailyMed |
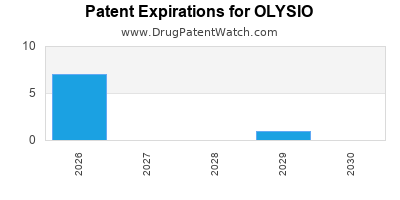

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for OLYSIO
Generic Entry Date for OLYSIO*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for OLYSIO
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Stanford University | Phase 4 |
| Yale University | Phase 4 |
| Alexion Pharmaceuticals | Phase 1 |
Anatomical Therapeutic Chemical (ATC) Classes for OLYSIO
US Patents and Regulatory Information for OLYSIO
OLYSIO is protected by nine US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of OLYSIO is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting OLYSIO
HCV NS-3 serine protease inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocylic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
Macrocyclic inhibitors of hepatitis C virus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: METHOD OF TREATING HEPATITIS C
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Prods | OLYSIO | simeprevir sodium | CAPSULE;ORAL | 205123-001 | Nov 22, 2013 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for OLYSIO
When does loss-of-exclusivity occur for OLYSIO?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
African Regional IP Organization (ARIPO)
Patent: 06
Estimated Expiration: ⤷ Try a Trial
Argentina
Patent: 5359
Estimated Expiration: ⤷ Try a Trial
Patent: 9345
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 06274865
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 94288
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0614654
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 16580
Estimated Expiration: ⤷ Try a Trial
China
Patent: 1228169
Estimated Expiration: ⤷ Try a Trial
Patent: 2627639
Estimated Expiration: ⤷ Try a Trial
Patent: 3030636
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 83
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0110237
Estimated Expiration: ⤷ Try a Trial
Patent: 0151326
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 12006
Estimated Expiration: ⤷ Try a Trial
Patent: 17392
Estimated Expiration: ⤷ Try a Trial
Patent: 14044
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 12999
Estimated Expiration: ⤷ Try a Trial
Patent: 22516
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 088150
Estimated Expiration: ⤷ Try a Trial
El Salvador
Patent: 08002642
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 5131
Estimated Expiration: ⤷ Try a Trial
Patent: 0800476
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 12999
Estimated Expiration: ⤷ Try a Trial
Patent: 22516
Estimated Expiration: ⤷ Try a Trial
Patent: 37339
Estimated Expiration: ⤷ Try a Trial
Germany
Patent: 2006019439
Estimated Expiration: ⤷ Try a Trial
Guatemala
Patent: 0600339
Estimated Expiration: ⤷ Try a Trial
Honduras
Patent: 08000134
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 16771
Estimated Expiration: ⤷ Try a Trial
Patent: 83872
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 27156
Estimated Expiration: ⤷ Try a Trial
Patent: 400054
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 8227
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 97067
Estimated Expiration: ⤷ Try a Trial
Patent: 09502889
Estimated Expiration: ⤷ Try a Trial
Luxembourg
Patent: 568
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 4217
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 231
Estimated Expiration: ⤷ Try a Trial
Patent: 415
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 4550
Estimated Expiration: ⤷ Try a Trial
Nicaragua
Patent: 0800036
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 2393
Estimated Expiration: ⤷ Try a Trial
Patent: 081073
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 070211
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 12999
Estimated Expiration: ⤷ Try a Trial
Patent: 22516
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 12999
Estimated Expiration: ⤷ Try a Trial
Patent: 22516
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 743
Estimated Expiration: ⤷ Try a Trial
Patent: 473
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 3617
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 12999
Estimated Expiration: ⤷ Try a Trial
Patent: 22516
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0800857
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1059419
Estimated Expiration: ⤷ Try a Trial
Patent: 080042084
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 60473
Estimated Expiration: ⤷ Try a Trial
Patent: 55230
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 58411
Estimated Expiration: ⤷ Try a Trial
Patent: 0745117
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 245
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 703
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering OLYSIO around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 4902361 | ⤷ Try a Trial | |
| Israel | 188227 | תרכובות מקרוציקליות, שילובים ותכשירים רוקחיים המכילים אותן, שימושים בהן כמעכבי וירוס הפטיטיס c ותהליכים להכנתן (Macrocyclic compounds, combinations and pharmaceutical compositions comprising them, uses thereof as inhibitors of hepatitis c virus and processes for their preparation) | ⤷ Try a Trial |
| Costa Rica | 9783 | INHIBIDORES MACROCICLICOS DEL VIRUS DE LA HEPATITIS C | ⤷ Try a Trial |
| Portugal | 2322516 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 2005073195 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for OLYSIO
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1912999 | 2014/058 | Ireland | ⤷ Try a Trial | PRODUCT NAME: SIMEPREVIR, OR A SALT THEREOF, INCLUDING SIMEPREVIR SODIUM; REGISTRATION NO/DATE: EU/1/14/924/001-002 20140516 |
| 1912999 | 1490062-5 | Sweden | ⤷ Try a Trial | PRODUCT NAME: SIMEPREVIR, OR A SALT THEREOF, INCLUDING SIMEPREVIR SODIUM; REG. NO/DATE: EU/1/14/924 20140516 |
| 1713823 | 300703 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: SIMEPREVIR, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN, WAARONDER SIMEPREVIRNATRIUM; REGISTRATION NO/DATE: EU/1/14/924/001-002 20140516 |
| 1713823 | 132014902308868 | Italy | ⤷ Try a Trial | PRODUCT NAME: SIMEPREVIR O UN SUO SALE FARMACEUTICAMENTE ACCETTABILE, COMPRESO IL SALE SODICO(OLYSIO); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/14/924, 20140516 |
| 1713823 | CA 2014 00059 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SIMEPREVIR ELLER ET FARMACEUTISK SALT DERAF, HERUNDER SIMEPREVIRNATRIUM; REG. NO/DATE: EU/1/14/924 20140514 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


