NUBEQA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Nubeqa, and what generic alternatives are available?
Nubeqa is a drug marketed by Bayer Healthcare and is included in one NDA. There are eight patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and twenty-two patent family members in thirty-six countries.
The generic ingredient in NUBEQA is darolutamide. One supplier is listed for this compound. Additional details are available on the darolutamide profile page.
DrugPatentWatch® Generic Entry Outlook for Nubeqa
Nubeqa was eligible for patent challenges on July 30, 2023.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be February 27, 2038. This may change due to patent challenges or generic licensing.
There is one Paragraph IV patent challenge for this drug. This may lead to patent invalidation or a license for generic production.
Indicators of Generic Entry
Summary for NUBEQA
| International Patents: | 122 |
| US Patents: | 8 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 46 |
| Clinical Trials: | 12 |
| Patent Applications: | 150 |
| Drug Prices: | Drug price information for NUBEQA |
| What excipients (inactive ingredients) are in NUBEQA? | NUBEQA excipients list |
| DailyMed Link: | NUBEQA at DailyMed |
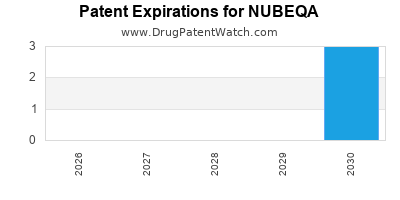

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for NUBEQA
Generic Entry Date for NUBEQA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for NUBEQA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Bayer | Phase 1/Phase 2 |
| Praful Ravi | Phase 1/Phase 2 |
| Eli Lilly and Company | Phase 1/Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for NUBEQA
Paragraph IV (Patent) Challenges for NUBEQA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| NUBEQA | Tablets | darolutamide | 300 mg | 212099 | 1 | 2023-07-31 |
US Patents and Regulatory Information for NUBEQA
NUBEQA is protected by eight US patents and two FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of NUBEQA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting NUBEQA
Carboxamide derivative and its diastereomers in stable crystalline form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Carboxamide derivative and its diastereomers in stable crystalline form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Androgen receptor modulating compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Carboxamide derivative and its diastereomers in stable crystalline form
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PATIENTS WITH NON-METASTATIC CASTRATION RESISTANT PROSTATE CANCER
Androgen receptor modulating compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Manufacture of a crystalline pharmaceutical product
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Androgen receptor modulating compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PATIENTS WITH NON-METASTATIC CASTRATION RESISTANT PROSTATE CANCER
Androgen receptor modulating compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PATIENTS WITH NON-METASTATIC CASTRATION RESISTANT PROSTATE CANCER
FDA Regulatory Exclusivity protecting NUBEQA
NEW CHEMICAL ENTITY
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF ADULT PATIENTS WITH METASTATIC HORMONE‐SENSITIVE PROSTATE CANCER (MHSPC) IN COMBINATION WITH DOCETAXEL
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bayer Healthcare | NUBEQA | darolutamide | TABLET;ORAL | 212099-001 | Jul 30, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Bayer Healthcare | NUBEQA | darolutamide | TABLET;ORAL | 212099-001 | Jul 30, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Bayer Healthcare | NUBEQA | darolutamide | TABLET;ORAL | 212099-001 | Jul 30, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Bayer Healthcare | NUBEQA | darolutamide | TABLET;ORAL | 212099-001 | Jul 30, 2019 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for NUBEQA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Bayer AG | Nubeqa | darolutamide | EMEA/H/C/004790 NUBEQA is indicated for the treatment of adult men with- non metastatic castration resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease (see section 5.1).- metastatic hormone sensitive prostate cancer (mHSPC) in combination with docetaxel and androgen deprivation therapy (see section 5.1). |
Authorised | no | no | no | 2020-03-27 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for NUBEQA
When does loss-of-exclusivity occur for NUBEQA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 18229817
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2019018458
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 55019
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 19002540
Estimated Expiration: ⤷ Try a Trial
Patent: 23002780
Estimated Expiration: ⤷ Try a Trial
China
Patent: 0382467
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1992103
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 92732
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 57071
Estimated Expiration: ⤷ Try a Trial
Patent: 20510018
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 19010452
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 190126111
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 6071
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering NUBEQA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 2021020935 | 安定結晶形のカルボキサミド誘導体およびそのジアステレオマー (CARBOXAMIDE DERIVATIVE AND ITS DIASTEREOMERS IN STABLE CRYSTALLINE FORM) | ⤷ Try a Trial |
| Eurasian Patent Organization | 201992103 | ПОЛУЧЕНИЕ КРИСТАЛЛИЧЕСКОГО ФАРМАЦЕВТИЧЕСКОГО ПРОДУКТА | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2007090624 | ⤷ Try a Trial | |
| Russian Federation | 2008136205 | ФУНГИЦИДНАЯ КОМПОЗИЦИЯ | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for NUBEQA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1986495 | CA 2020 00009 | Denmark | ⤷ Try a Trial | PRODUCT NAME: SEDAXAN ELLER EN TAUTOMER DERAF, FLUDIOXONIL OG METALAXYL M; NAT. REG. NO/DATE: 1-235 20191015; FIRST REG. NO/DATE: NL 15544 N 20171229 |
| 2493858 | SPC/GB20/022 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: DAROLUTAMIDE OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT OR ESTER THEREOF; REGISTERED: UK EU/1/20/1432/001(NI) 20200330; UK PLGB 00010/0677 20200330 |
| 2493858 | 301041 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: DAROLUTAMIDE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN OF IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBARE ESTER DAARVAN; REGISTRATION NO/DATE: EU/1/20/1432 20200330 |
| 2493858 | 2020018 | Norway | ⤷ Try a Trial | PRODUCT NAME: DAROLUTAMID, EVENTUELT I FORM AV ET FARMASOEYTISK AKSEPTABELT SALT ELLER ESTER DERAV; REG. NO/DATE: EU/1/20/1432/ 20200401 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


