MAVYRET Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Mavyret, and when can generic versions of Mavyret launch?
Mavyret is a drug marketed by Abbvie and is included in two NDAs. There are ten patents protecting this drug.
This drug has five hundred and thirty patent family members in forty-six countries.
The generic ingredient in MAVYRET is glecaprevir; pibrentasvir. One supplier is listed for this compound. Additional details are available on the glecaprevir; pibrentasvir profile page.
DrugPatentWatch® Generic Entry Outlook for Mavyret
Mavyret was eligible for patent challenges on August 3, 2021.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be December 5, 2035. This may change due to patent challenges or generic licensing.
Indicators of Generic Entry
Summary for MAVYRET
| International Patents: | 530 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 29 |
| Drug Prices: | Drug price information for MAVYRET |
| What excipients (inactive ingredients) are in MAVYRET? | MAVYRET excipients list |
| DailyMed Link: | MAVYRET at DailyMed |
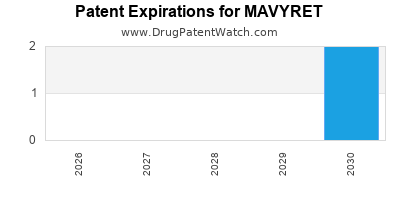
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for MAVYRET
Generic Entry Dates for MAVYRET*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
Generic Entry Dates for MAVYRET*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
PELLETS;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for MAVYRET
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| White River Junction Veterans Affairs Medical Center | Phase 2 |
| White River Junction Veterans Affairs Medical Center | Phase 2/Phase 3 |
| Duke University | Phase 4 |
Pharmacology for MAVYRET
Anatomical Therapeutic Chemical (ATC) Classes for MAVYRET
US Patents and Regulatory Information for MAVYRET
MAVYRET is protected by ten US patents and five FDA Regulatory Exclusivities.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of MAVYRET is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting MAVYRET
Anti-viral compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Anti-viral compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Method for treating HCV
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Solid pharmaceutical compositions for treating HCV
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Macrocyclic proline derived HCV serine protease inhibitors
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Anti-viral compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystal forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Anti-viral compounds
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystal forms
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
FDA Regulatory Exclusivity protecting MAVYRET
FOR TREATMENT OF PEDIATRIC PATIENTS 3 YEARS OF AGE TO LESS THAN 12 YEARS OF AGE WEIGHING LESS THAN 45 KG WITH CHRONIC HEPATITIS C VIRUS (HCV) GENOTYPE 1, 2, 3, 4, 5 OR 6 INFECTION WITHOUT CIRRHOSIS OR WITH COMPENSATED CIRRHOSIS (CHILD-PUGH A); AND TREATMENT OF PEDIATRIC PATIENTS 3 YEARS OF AGE TO LESS THAN 12 YEARS OF AGE WEIGHING LESS THAN 45 KG WITH HCV GENOTYPE 1 INFECTION, WHO PREVIOUSLY HAVE BEEN TREATED WITH A REGIMEN CONTAINING AN HCV NS5A INHIBITOR OR AN NS3/4A PROTEASE INHIBITOR (PI), BUT NOT BOTH
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF PEDIATRIC PATIENTS 12 YEARS AND OLDER OR WEIGHING AT LEAST 45 KG WITH CHRONIC HEPATITIS C VIRUS (HCV) GENOTYPE 1,2,3,4,5 OR 6 INFECTION WITHOUT CIRRHOSIS OR WITH COMPENSATED CIRRHOSIS (CHILD-PUGH A)
Exclusivity Expiration: ⤷ Try a Trial
TREATMENT OF PEDIATRIC PATIENTS 12 YEARS AND OLDER OR WEIGHING AT LEAST 45 KG WITH HCV GENOTYPE 1 INFECTION, WHO PREVIOUSLY HAVE BEEN TREATED WITH A REGIMEN CONTAINING AN HCV NS5A INHIBITOR OR AN NS3/4A PROTEASE INHIBITOR (PI), BUT NOT BOTH
Exclusivity Expiration: ⤷ Try a Trial
PEDIATRIC EXCLUSIVITY
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | PELLETS;ORAL | 215110-001 | Jun 10, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | TABLET;ORAL | 209394-001 | Aug 3, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | TABLET;ORAL | 209394-001 | Aug 3, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | PELLETS;ORAL | 215110-001 | Jun 10, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | PELLETS;ORAL | 215110-001 | Jun 10, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | TABLET;ORAL | 209394-001 | Aug 3, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Abbvie | MAVYRET | glecaprevir; pibrentasvir | TABLET;ORAL | 209394-001 | Aug 3, 2017 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for MAVYRET
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| AbbVie Deutschland GmbH Co. KG | Maviret | glecaprevir, pibrentasvir | EMEA/H/C/004430 Maviret is indicated for the treatment of chronic hepatitis C virus (HCV) infection in adults and children aged 3 years and older.Maviret coated granules is indicated for the treatment of chronic hepatitis C virus (HCV) infection in children 3 years and older. |
Authorised | no | no | no | 2017-07-26 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for MAVYRET
When does loss-of-exclusivity occur for MAVYRET?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 15269306
Estimated Expiration: ⤷ Try a Trial
Patent: 16283018
Estimated Expiration: ⤷ Try a Trial
Patent: 16296709
Estimated Expiration: ⤷ Try a Trial
Patent: 20239679
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2017028185
Estimated Expiration: ⤷ Try a Trial
Patent: 2018000982
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 48902
Estimated Expiration: ⤷ Try a Trial
Patent: 90855
Estimated Expiration: ⤷ Try a Trial
Patent: 92722
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 17003350
Estimated Expiration: ⤷ Try a Trial
Patent: 18000138
Estimated Expiration: ⤷ Try a Trial
China
Patent: 6413736
Estimated Expiration: ⤷ Try a Trial
Patent: 7920996
Estimated Expiration: ⤷ Try a Trial
Patent: 8024964
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 17013305
Estimated Expiration: ⤷ Try a Trial
Patent: 18000391
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 180030
Estimated Expiration: ⤷ Try a Trial
Patent: 180088
Estimated Expiration: ⤷ Try a Trial
Dominican Republic
Patent: 017000314
Estimated Expiration: ⤷ Try a Trial
Patent: 018000024
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 18000689
Estimated Expiration: ⤷ Try a Trial
Patent: 18008411
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 1890160
Estimated Expiration: ⤷ Try a Trial
Patent: 1890334
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 51850
Estimated Expiration: ⤷ Try a Trial
Patent: 13378
Estimated Expiration: ⤷ Try a Trial
Patent: 24941
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 50627
Estimated Expiration: ⤷ Try a Trial
Patent: 55203
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6504
Estimated Expiration: ⤷ Try a Trial
Patent: 6945
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 33466
Estimated Expiration: ⤷ Try a Trial
Patent: 62425
Estimated Expiration: ⤷ Try a Trial
Patent: 17518319
Estimated Expiration: ⤷ Try a Trial
Patent: 18518517
Estimated Expiration: ⤷ Try a Trial
Patent: 18520185
Estimated Expiration: ⤷ Try a Trial
Patent: 21113192
Estimated Expiration: ⤷ Try a Trial
Patent: 22141719
Estimated Expiration: ⤷ Try a Trial
Patent: 22177014
Estimated Expiration: ⤷ Try a Trial
Patent: 23089125
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 2606
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 16016127
Estimated Expiration: ⤷ Try a Trial
Patent: 18000218
Estimated Expiration: ⤷ Try a Trial
Patent: 18000746
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 180488
Estimated Expiration: ⤷ Try a Trial
Patent: 180609
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 017502426
Estimated Expiration: ⤷ Try a Trial
Patent: 018500132
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 18102809
Estimated Expiration: ⤷ Try a Trial
Patent: 18105849
Estimated Expiration: ⤷ Try a Trial
Patent: 21102950
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 202002899V
Estimated Expiration: ⤷ Try a Trial
Patent: 202002900Y
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1800533
Estimated Expiration: ⤷ Try a Trial
Patent: 1801082
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2637828
Estimated Expiration: ⤷ Try a Trial
Patent: 180021840
Estimated Expiration: ⤷ Try a Trial
Patent: 180025317
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering MAVYRET around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Malaysia | 164607 | SOLID COMPOSITIONS | ⤷ Try a Trial |
| Serbia | 56202 | KOMBINACIJA DVA ANTIVIROTIKA ZA LEČENJE HEPATITISA C (COMBINATION OF TWO ANTIVIRALS FOR TREATING HEPATITIS C) | ⤷ Try a Trial |
| Portugal | 2692346 | ⤷ Try a Trial | |
| Mexico | 2016014459 | FORMAS DE CRISTAL. (CRYSTAL FORMS.) | ⤷ Try a Trial |
| Luxembourg | C00037 | ⤷ Try a Trial | |
| World Intellectual Property Organization (WIPO) | 2017040766 | ⤷ Try a Trial | |
| European Patent Office | 3313378 | COMPOSITIONS PHARMACEUTIQUES SOLIDES POUR LE TRAITEMENT DU VHC (SOLID PHARMACEUTICAL COMPOSITIONS FOR TREATING HCV) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for MAVYRET
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2692346 | 1790050-7/1791051-4 | Sweden | ⤷ Try a Trial | PRODUCT NAME: PIBRENTASVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT THEREOF. |
| 2692346 | 17C1040 | France | ⤷ Try a Trial | PRODUCT NAME: PIBRENTASVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; REGISTRATION NO/DATE: EU/1/17/1213 20170728 |
| 2692346 | 51/2017 | Austria | ⤷ Try a Trial | PRODUCT NAME: PIBRENTASVIR ODER EIN PHARMAZEUTISCH ANNEHMBARES SALZ DAVON; REGISTRATION NO/DATE: EU/1/17/1213 (MITTEILUNG) 20170728 |
| 2618831 | 300900 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: GLECAPREVIR OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF ESTER DAARVAN; REGISTRATION NO/DATE: EU/1/17/1213 20170728 |
| 2368890 | 92668 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: OMBITASVIR, OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE QUI EN DERIVE (VIEKIRAX R); FIRST REGISTRATION DATE: 20150119 |
| 2692346 | 299 50017-2017 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: PIBRENTASVIR VO VSETKYCH FORMACH CHRANENYCH ZAKLADNYM PATENTOM; REGISTRATION NO/DATE: EU/1/17/1213 20170728 |
| 2618831 | SPC/GB17/057 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: GLECAPREVIR OR A PHARMACEUTICALLY ACCEPTABLE SALT OR ESTER THEREOF; REGISTERED: UK EU/1/17/1213 (NI) 20170728; UK FURTHER MAS ON IPSUM 20170728 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


