JEVTANA KIT Drug Patent Profile
✉ Email this page to a colleague
When do Jevtana Kit patents expire, and what generic alternatives are available?
Jevtana Kit is a drug marketed by Sanofi Aventis Us and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has ninety-six patent family members in forty-six countries.
The generic ingredient in JEVTANA KIT is cabazitaxel. There are thirteen drug master file entries for this compound. One supplier is listed for this compound. Additional details are available on the cabazitaxel profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Jevtana Kit
A generic version of JEVTANA KIT was approved as cabazitaxel by ACCORD HLTHCARE on December 29th, 2021.
Summary for JEVTANA KIT
| International Patents: | 96 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 73 |
| Clinical Trials: | 54 |
| Patent Applications: | 1,915 |
| Formulation / Manufacturing: | see details |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for JEVTANA KIT |
| DailyMed Link: | JEVTANA KIT at DailyMed |
Recent Clinical Trials for JEVTANA KIT
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Andrew J. Armstrong, MD | Phase 2 |
| Janssen Pharmaceutica | Phase 2 |
| Oregon Health and Science University | Phase 1 |
Anatomical Therapeutic Chemical (ATC) Classes for JEVTANA KIT
Paragraph IV (Patent) Challenges for JEVTANA KIT
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| JEVTANA KIT | Injection | cabazitaxel | 60 mg/1.5 mL | 201023 | 8 | 2014-06-17 |
US Patents and Regulatory Information for JEVTANA KIT
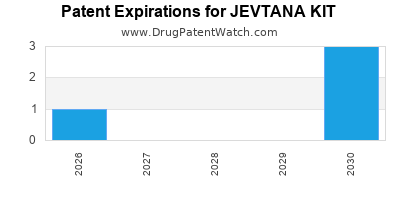
JEVTANA KIT is protected by four US patents.
Patents protecting JEVTANA KIT
Antitumoral use of cabazitaxel
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: INCREASING SURVIVAL IN METASTATIC CASTRATION-RESISTANT PROSTATE CANCER PATIENTS PREVIOUSLY TREATED WITH DOCETAXEL BY ADMINISTERING AS A 3 WEEK CYCLE CABAZITAXEL AFTER 5 MG DEXCHLORPHENIRAMINE, 8 MG DEXAMETHASONE, AND AN H2-AGONIST
Antitumoral use of cabazitaxel
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: INCREASING SURVIVAL IN METASTATIC CASTRATION-RESISTANT PROSTATE CANCER PATIENTS PREVIOUSLY TREATED WITH DOCETAXEL BY ADMINISTERING 20 TO 25 MG/M2 CABAZITAXEL AFTER A PREMEDICATION REGIMEN THAT INCLUDES AN H2-ANTAGONIST
Acetone solvate of dimethoxy docetaxel and its process of preparation
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Antitumoral use of cabazitaxel
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | AP | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for JEVTANA KIT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for JEVTANA KIT
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Accord Healthcare S.L.U. | Cabazitaxel Accord | cabazitaxel | EMEA/H/C/005178 Treatment of patients with hormone refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen. |
Authorised | yes | no | no | 2020-08-28 | |
| Sanofi Winthrop Industrie | Jevtana | cabazitaxel | EMEA/H/C/002018 Jevtana in combination with prednisone or prednisolone is indicated for the treatment of patients with hormone-refractory metastatic prostate cancer previously treated with a docetaxel-containing regimen. |
Authorised | no | no | no | 2011-03-17 | |
| Teva B.V. | Cabazitaxel Teva | cabazitaxel | EMEA/H/C/004951 Treatment of prostate cancer |
Refused | no | no | no | 2019-07-11 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for JEVTANA KIT
See the table below for patents covering JEVTANA KIT around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Czech Republic | 284080 | Injikovatelná kompozice obsahující deriváty skupiny taxanů (INJECTABLE COMPOSITION INTENDED FOR PREPARATION OF INFUSION SOLUTION) | ⤷ Try a Trial |
| European Patent Office | 0522937 | ⤷ Try a Trial | |
| Canada | 2779009 | NOUVELLE UTILISATION ANTITUMORALE DU CABAZITAXEL (NOVEL ANTITUMORAL USE OF CABAZITAXEL) | ⤷ Try a Trial |
| Hungary | 222833 | Taxánszármazékot tartalmazó injektálható gyógyszerkészítmények (INJECTABLE MEDICAMENTS CONTAINING TAXANE DERIVATIVES) | ⤷ Try a Trial |
| Panama | 8612401 | SOLVATO ACETONICO DEL DIMETOXI DOCETAXEL Y SU PROCEDIMIENTO DE PREPARACION | ⤷ Try a Trial |
| Hungary | 223732 | Taxoidok, eljárás előállításukra és ezeket tartalmazó gyógyszerkészítmények (TAXOIDS, PREPARATION THEREOF AND PHARMACEUTICALS CONTAINING SAME) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for JEVTANA KIT
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1667986 | 2013/021 | Ireland | ⤷ Try a Trial | PRODUCT NAME: CABAZITAXEL ACETONE SOLVATE; REGISTRATION NO/DATE: EU/1/11/676/001 20110317 |
| 1667986 | C300595 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: CABAZITAXEL ACETONAAT; REGISTRATION NO/DATE: EU/1/11/676/001 20110317 |
| 1667986 | 13C0037 | France | ⤷ Try a Trial | PRODUCT NAME: CABAZITAXEL SOUS FORME ACETONATE (OU SOLVAT ACETONIQUE DU CABAZITAXEL); REGISTRATION NO/DATE: EU/1/11/676/001 20110322 |
| 1667986 | C01667986/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: CABAZITAXELUM; REGISTRATION NO/DATE: SWISSMEDIC 61543 06.04.2011 |
| 1667986 | 636 | Finland | ⤷ Try a Trial | |
| 1667986 | CA 2013 00025 | Denmark | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


