INVELTYS Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Inveltys, and when can generic versions of Inveltys launch?
Inveltys is a drug marketed by Alcon Labs Inc and is included in one NDA. There are twelve patents protecting this drug.
This drug has eighty-four patent family members in twelve countries.
The generic ingredient in INVELTYS is loteprednol etabonate. There are ten drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the loteprednol etabonate profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Inveltys
A generic version of INVELTYS was approved as loteprednol etabonate by SENTISS on April 17th, 2019.
Summary for INVELTYS
| International Patents: | 84 |
| US Patents: | 12 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 63 |
| Patent Applications: | 4,910 |
| Formulation / Manufacturing: | see details |
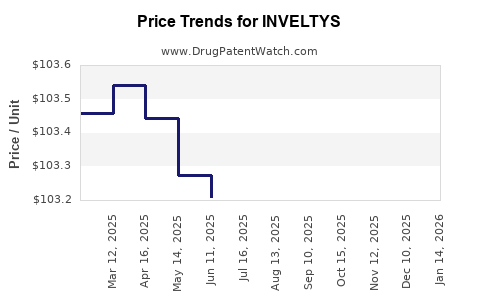
| Drug Prices: | Drug price information for INVELTYS |
| What excipients (inactive ingredients) are in INVELTYS? | INVELTYS excipients list |
| DailyMed Link: | INVELTYS at DailyMed |
Pharmacology for INVELTYS
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for INVELTYS
US Patents and Regulatory Information for INVELTYS
INVELTYS is protected by thirteen US patents.
Patents protecting INVELTYS
Nanocrystals, compositions, and methods that aid particle transport in mucus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR DELIVERING A PHARMACEUTICAL AGENT ACROSS A MUCOSAL BARRIER
Compositions and methods for ophthalmic and/or other applications
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for ophthalmic and/or other applications
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Compositions and methods for ophthalmic and/or other applications
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR TREATING OCULAR INFLAMMATION
Compositions and methods for ophthalmic and/or other applications
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF REDUCING POST-SURGICAL PAIN FOLLOWING OCULAR SURGERY
Compositions and methods for ophthalmic and/or other applications
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF TREATING POSTOPERATIVE INFLAMMATION FOLLOWING OCULAR SURGERY
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Nanocrystals, compositions, and methods that aid particle transport in mucus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR DELIVERING A COMPOSITION TO A MUCUS MEMBRANE
Nanocrystals, compositions, and methods that aid particle transport in mucus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Nanocrystals, compositions, and methods that aid particle transport in mucus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR DELIVERING A COMPOSITION TO A MUCUS MEMBRANE
Nanocrystals, compositions, and methods that aid particle transport in mucus
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR DELIVERING A PHARMACEUTICAL AGENT ACROSS A MUCOSAL BARRIER
Compositions and methods for ophthalmic and/or other applications
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD FOR TREATING INFLAMMATION AND/OR OTHER DISORDERS IN AN EYE OF A PATIENT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Alcon Labs Inc | INVELTYS | loteprednol etabonate | SUSPENSION/DROPS;OPHTHALMIC | 210565-001 | Aug 22, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for INVELTYS
See the table below for patents covering INVELTYS around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20150006868 | PHARMACEUTICAL NANOPARTICLES SHOWING IMPROVED MUCOSAL TRANSPORT | ⤷ Try a Trial |
| Japan | 7320219 | ⤷ Try a Trial | |
| South Korea | 102154880 | ⤷ Try a Trial | |
| Japan | 2018162284 | 複数の被覆された粒子を含む組成物、医薬組成物、医薬製剤、及び当該粒子の形成方法 (COMPOSITIONS COMPRISING MULTIPLE COATED PARTICLES, PHARMACEUTICAL COMPOSITIONS, PHARMACEUTICAL FORMULATIONS, AND METHODS OF FORMING THOSE PARTICLES) | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2015066444 | ⤷ Try a Trial | |
| China | 111700878 | 显示提高的粘膜转移的药物纳米粒子 (PHARMACEUTICAL NANOPARTICLES SHOWING IMPROVED MUCOSAL TRANSPORT) | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |


