GALAFOLD Drug Patent Profile
✉ Email this page to a colleague
When do Galafold patents expire, and what generic alternatives are available?
Galafold is a drug marketed by Amicus Therap Us and is included in one NDA. There are fifty-eight patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and twenty-one patent family members in twenty-nine countries.
The generic ingredient in GALAFOLD is migalastat hydrochloride. One supplier is listed for this compound. Additional details are available on the migalastat hydrochloride profile page.
DrugPatentWatch® Generic Entry Outlook for Galafold
Galafold was eligible for patent challenges on August 10, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be August 10, 2025. This may change due to patent challenges or generic licensing.
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for GALAFOLD
| International Patents: | 221 |
| US Patents: | 58 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 41 |
| Clinical Trials: | 10 |
| Patent Applications: | 109 |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for GALAFOLD |
| What excipients (inactive ingredients) are in GALAFOLD? | GALAFOLD excipients list |
| DailyMed Link: | GALAFOLD at DailyMed |
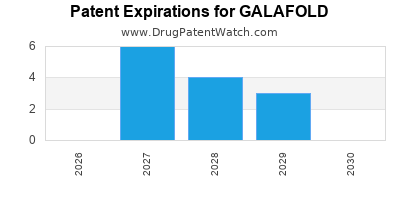
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for GALAFOLD
Generic Entry Date for GALAFOLD*:
Constraining patent/regulatory exclusivity:
INDICATED FOR THE TREATMENT OF ADULTS WITH A CONFIRMED DIAGNOSIS OF FABRY DISEASE AND AN AMENABLE GALACTOSIDASE ALPHA GENE (GLA) VARIANT BASED ON IN VITRO ASSAY DATA NDA:
Dosage:
CAPSULE;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for GALAFOLD
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Amicus Therapeutics | Phase 3 |
| Amicus Therapeutics | Phase 2 |
Anatomical Therapeutic Chemical (ATC) Classes for GALAFOLD
Paragraph IV (Patent) Challenges for GALAFOLD
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| GALAFOLD | Capsules | migalastat hydrochloride | 123 mg | 208623 | 3 | 2022-08-10 |
US Patents and Regulatory Information for GALAFOLD
GALAFOLD is protected by fifty-nine US patents and one FDA Regulatory Exclusivity.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of GALAFOLD is ⤷ Try a Trial.
This potential generic entry date is based on INDICATED FOR THE TREATMENT OF ADULTS WITH A CONFIRMED DIAGNOSIS OF FABRY DISEASE AND AN AMENABLE GALACTOSIDASE ALPHA GENE (GLA) VARIANT BASED ON IN VITRO ASSAY DATA.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting GALAFOLD
Methods of treating fabry disease in patients having the G9331A mutation in the GLA gene
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods for treatment of Fabry disease
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods for treatment of fabry disease
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Method to predict response to pharmacological chaperone treatment of diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Use of migalastat for treating Fabry disease in pregnant patients
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Methods of treating fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods of treating Fabry patients having renal impairment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Method to predict response to pharmacological chaperone treatment of diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods for treatment of Fabry disease
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Method to predict response to pharmacological chaperone treatment of diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods for treatment of Fabry disease
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Methods for treatment of Fabry disease
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF REDUCING PODOCYTE GLOBOTRIAOSYLCERAMIDE (GL-3) IN A FABRY PATIENT BY ADMINISTERING MIGALASTAT
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: A METHOD OF REDUCING LEFT VENTRICULAR MASS INDEX (LVMI) IN A FABRY PATIENT BY ADMINISTERING MIGALASTAT
Method to predict response to pharmacological chaperone treatment of diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: THE TREATMENT OF FABRY PATIENTS
FDA Regulatory Exclusivity protecting GALAFOLD
INDICATED FOR THE TREATMENT OF ADULTS WITH A CONFIRMED DIAGNOSIS OF FABRY DISEASE AND AN AMENABLE GALACTOSIDASE ALPHA GENE (GLA) VARIANT BASED ON IN VITRO ASSAY DATA
Exclusivity Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amicus Therap Us | GALAFOLD | migalastat hydrochloride | CAPSULE;ORAL | 208623-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amicus Therap Us | GALAFOLD | migalastat hydrochloride | CAPSULE;ORAL | 208623-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Amicus Therap Us | GALAFOLD | migalastat hydrochloride | CAPSULE;ORAL | 208623-001 | Aug 10, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for GALAFOLD
See the table below for patents covering GALAFOLD around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| South Korea | 20240017111 | 신장 손상을 갖는 파브리 환자를 치료하는 방법 (METHODS OF TREATING FABRY PATIENTS HAVING RENAL IMPAIRMENT) | ⤷ Try a Trial |
| Chile | 2022002423 | Métodos para tratar la enfermedad de fabry | ⤷ Try a Trial |
| Portugal | 2252313 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for GALAFOLD
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2787345 | 53/2016 | Austria | ⤷ Try a Trial | PRODUCT NAME: MIGALASTAT ODER EIN SALZ DAVON, EINSCHLIESSLICH DES HYDROCHLORIDSALZES; REGISTRATION NO/DATE: EU/1/15/1082 (MITTEILUNG) 20160531 |
| 2787345 | 16C1014 | France | ⤷ Try a Trial | PRODUCT NAME: MIGALASTAT OU UN SEL DE CELUI-CI,NOTAMENT LE CHLORHYDRATE; REGISTRATION NO/DATE: EU1/15/1082 20160531 |
| 2787345 | C 2016 042 | Romania | ⤷ Try a Trial | PRODUCT NAME: MIGALASTAT SAU O SARE A ACESTUIA, INCLUSIV SAREACLORHIDRAT; NATIONAL AUTHORISATION NUMBER: EU/1/15/1082; DATE OF NATIONAL AUTHORISATION: 20160526; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/15/1082; DATE OF FIRST AUTHORISATION IN EEA: 20160526 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |


