FYCOMPA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Fycompa, and when can generic versions of Fycompa launch?
Fycompa is a drug marketed by Catalyst Pharms and is included in two NDAs. There are two patents protecting this drug and two Paragraph IV challenges.
This drug has one hundred and one patent family members in thirty-three countries.
The generic ingredient in FYCOMPA is perampanel. There are five drug master file entries for this compound. Two suppliers are listed for this compound. Additional details are available on the perampanel profile page.
DrugPatentWatch® Generic Entry Outlook for Fycompa
Fycompa was eligible for patent challenges on October 22, 2016.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be July 1, 2026. This may change due to patent challenges or generic licensing.
There has been one patent litigation case involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
There are three tentative approvals for the generic drug (perampanel), which indicates the potential for near-term generic launch.
Indicators of Generic Entry
Summary for FYCOMPA
| International Patents: | 101 |
| US Patents: | 2 |
| Applicants: | 1 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 2 |
| Raw Ingredient (Bulk) Api Vendors: | 59 |
| Clinical Trials: | 22 |
| Patent Applications: | 372 |
| Drug Prices: | Drug price information for FYCOMPA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for FYCOMPA |
| What excipients (inactive ingredients) are in FYCOMPA? | FYCOMPA excipients list |
| DailyMed Link: | FYCOMPA at DailyMed |
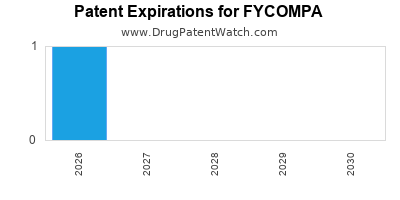
DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for FYCOMPA
Generic Entry Dates for FYCOMPA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
SUSPENSION;ORAL |
Generic Entry Dates for FYCOMPA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for FYCOMPA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Wayne State University | Phase 4 |
| Eisai Korea Inc. | Phase 4 |
| University of Florida | Phase 4 |
Pharmacology for FYCOMPA
Anatomical Therapeutic Chemical (ATC) Classes for FYCOMPA
Paragraph IV (Patent) Challenges for FYCOMPA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| FYCOMPA | Oral Suspension | perampanel | 0.5 mg/mL | 208277 | 1 | 2022-12-20 |
| FYCOMPA | Tablets | perampanel | 2 mg, 4 mg, 6 mg, 8 mg, 10 mg and 12 mg | 202834 | 2 | 2016-10-24 |
US Patents and Regulatory Information for FYCOMPA
FYCOMPA is protected by six US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of FYCOMPA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting FYCOMPA
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES WITH OR WITHOUT SECONDARILY GENERALIZED SEIZURES IN PATIENTS WITH EPILEPSY 4 YEARS OF AGE AND OLDER
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PARTIAL-ONSET SEIZURES WITH OR WITHOUT SECONDARILY GENERALIZED SEIZURES IN PATIENTS WITH EPILEPSY
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PRIMARY GENERALIZED TONIC-CLONIC SEIZURES AS ADJUNCTIVE THERAPY IN PATIENTS WITH EPILEPSY
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF EPILEPSY
1,2-dihydropyridine compounds, process for preparation of the same and use thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PRIMARY GENERALIZED TONIC-CLONIC SEIZURES AS ADJUNCTIVE THERAPY IN PATIENTS WITH EPILEPSY 12 YEARS OF AGE AND OLDER
Method for producing 1, 2-dihydropyridine-2-one compound
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catalyst Pharms | FYCOMPA | perampanel | SUSPENSION;ORAL | 208277-001 | Apr 29, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-002 | Oct 22, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-003 | Oct 22, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-001 | Oct 22, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-005 | Oct 22, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Catalyst Pharms | FYCOMPA | perampanel | TABLET;ORAL | 202834-001 | Oct 22, 2012 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for FYCOMPA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Eisai GmbH | Fycompa | perampanel | EMEA/H/C/002434 Fycompa is indicated for the adjunctive treatment of partial-onset seizures with or without secondarily generalised seizures in adult and adolescent patients from 12 years of age with epilepsy.Fycompa is indicated for the adjunctive treatment of primary generalised tonic-clonic seizures in adult and adolescent patients from 12 years of age with idiopathic generalised epilepsy. |
Authorised | no | no | no | 2012-07-23 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for FYCOMPA
When does loss-of-exclusivity occur for FYCOMPA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 05258378
Estimated Expiration: ⤷ Try a Trial
Patent: 05258385
Estimated Expiration: ⤷ Try a Trial
Austria
Patent: 47405
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 0510254
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 61829
Estimated Expiration: ⤷ Try a Trial
Patent: 70177
Estimated Expiration: ⤷ Try a Trial
China
Patent: 42443
Estimated Expiration: ⤷ Try a Trial
Patent: 84890
Estimated Expiration: ⤷ Try a Trial
Patent: 1544599
Estimated Expiration: ⤷ Try a Trial
Patent: 1914057
Estimated Expiration: ⤷ Try a Trial
Patent: 2382047
Estimated Expiration: ⤷ Try a Trial
Patent: 4402807
Estimated Expiration: ⤷ Try a Trial
Patent: 4402808
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0130363
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 14313
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 64361
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 64361
Estimated Expiration: ⤷ Try a Trial
Patent: 72450
Estimated Expiration: ⤷ Try a Trial
Patent: 86771
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 02127
Estimated Expiration: ⤷ Try a Trial
Patent: 04999
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 0024
Estimated Expiration: ⤷ Try a Trial
Patent: 0151
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 2006004100
Estimated Expiration: ⤷ Try a Trial
Patent: 2006004107
Estimated Expiration: ⤷ Try a Trial
Patent: 30206
Estimated Expiration: ⤷ Try a Trial
Patent: 19944
Estimated Expiration: ⤷ Try a Trial
Patent: 07099781
Estimated Expiration: ⤷ Try a Trial
Patent: 12021029
Estimated Expiration: ⤷ Try a Trial
Jordan
Patent: 32
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 8809
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 06014458
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 520
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 0720
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 9101
Estimated Expiration: ⤷ Try a Trial
Patent: 065754
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 64361
Estimated Expiration: ⤷ Try a Trial
Patent: 72450
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 64361
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 23930
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 752
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 64361
Estimated Expiration: ⤷ Try a Trial
Patent: 72450
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 0609274
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 0939743
Estimated Expiration: ⤷ Try a Trial
Patent: 1187218
Estimated Expiration: ⤷ Try a Trial
Patent: 070008617
Estimated Expiration: ⤷ Try a Trial
Patent: 070028459
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 04697
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 46660
Estimated Expiration: ⤷ Try a Trial
Patent: 0606144
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering FYCOMPA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Luxembourg | 92113 | ⤷ Try a Trial | |
| Hungary | 0303398 | ⤷ Try a Trial | |
| European Patent Office | 2177520 | ⤷ Try a Trial | |
| European Patent Office | 1300396 | COMPOSES 1,2-DIHYDROPYRIDINE, LEUR PROCEDE DE PREPARATION ET LEUR UTILISATION (1,2-DIHYDROPYRIDINE COMPOUNDS, PROCESS FOR PREPARATION OF THE SAME AND USE THEREOF) | ⤷ Try a Trial |
| Japan | 5419944 | ⤷ Try a Trial | |
| Norway | 339101 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for FYCOMPA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1764361 | C 2013 025 | Romania | ⤷ Try a Trial | PRODUCT NAME: PERAMPANEL3-(2-CIANOFENIL)-5-(2-PIRIDIL)-1-FENIL-1,2-DIHIDROPIRIDIN-2-ONA; NATIONAL AUBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/12/776/001 - EU/1/12/776/023; DATE OF FIRST AUTHORISATION IN EEA: 20120723 THORISATION NUMBER: EU/1/12/776/001 - EU/1/12/776/023; DATE OF NATIONAL AUTHORISATION: 20120723; NUM |
| 1764361 | 173 5-2013 | Slovakia | ⤷ Try a Trial | PRODUCT NAME: PERAMPANEL; NAT. REGISTRATION NO/DATE: EU/1/12/776/001 - EU/1/12/776/016 20120723; FIRST REGISTRATION: EU EU/1/12/776/001 - EU/1 /12/776/016 20120723 |
| 1300396 | 2012/049 | Ireland | ⤷ Try a Trial | PRODUCT NAME: PERAMPANEL, OPTIONALLY A SALT OR HYDRATE THEREOF.; REGISTRATION NO/DATE: EU/1/12/776/001-EU/1/12/776/016 20120723 |
| 1300396 | 92113 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: PERAMPANEL ET SES DERIVES PHARMACEUTIQUEMENT ACCEPTABLES (FYCOMPA) |
| 1764361 | PA2013017 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: PERAMPANELUM; REGISTRATION NO/DATE: EU/1/12/776/001 - EU/1/12/776/016, 2012 07 23 EU/1/12/776/017 - EU1/12/776/023 20121029 |
| 1300396 | 12C0074 | France | ⤷ Try a Trial | PRODUCT NAME: PERAMPANEL, SEL DE CELUI-CI OU HYDRATE DE CELUI-CI; REGISTRATION NO/DATE: EU/1/12/776/001 20120723 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


