ERLEADA Drug Patent Profile
✉ Email this page to a colleague
When do Erleada patents expire, and when can generic versions of Erleada launch?
Erleada is a drug marketed by Janssen Biotech and is included in one NDA. There are ten patents protecting this drug and one Paragraph IV challenge.
This drug has two hundred and sixty-five patent family members in forty-six countries.
The generic ingredient in ERLEADA is apalutamide. One supplier is listed for this compound. Additional details are available on the apalutamide profile page.
DrugPatentWatch® Generic Entry Outlook for Erleada
Erleada was eligible for patent challenges on February 14, 2022.
By analyzing the patents and regulatory protections it appears that the earliest date
for generic entry will be June 4, 2033. This may change due to patent challenges or generic licensing.
There have been five patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for ERLEADA
| International Patents: | 265 |
| US Patents: | 10 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 84 |
| Clinical Trials: | 20 |
| Patent Applications: | 724 |
| Drug Prices: | Drug price information for ERLEADA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for ERLEADA |
| What excipients (inactive ingredients) are in ERLEADA? | ERLEADA excipients list |
| DailyMed Link: | ERLEADA at DailyMed |
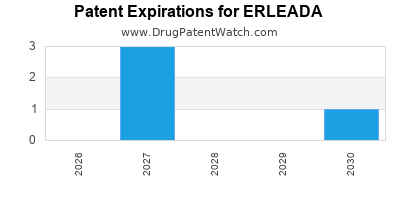

DrugPatentWatch® Estimated Loss of Exclusivity (LOE) Date for ERLEADA
Generic Entry Date for ERLEADA*:
Constraining patent/regulatory exclusivity:
NDA:
Dosage:
TABLET;ORAL |
*The generic entry opportunity date is the latter of the last compound-claiming patent and the last regulatory exclusivity protection. Many factors can influence early or later generic entry. This date is provided as a rough estimate of generic entry potential and should not be used as an independent source.
Recent Clinical Trials for ERLEADA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| National Cancer Institute (NCI) | Phase 1 |
| Janssen-Cilag Ltd. | Phase 3 |
| Cambridge University Hospitals NHS Foundation Trust | Phase 3 |
Anatomical Therapeutic Chemical (ATC) Classes for ERLEADA
Paragraph IV (Patent) Challenges for ERLEADA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| ERLEADA | Tablets | apalutamide | 60 mg | 210951 | 5 | 2022-02-14 |
US Patents and Regulatory Information for ERLEADA
ERLEADA is protected by fourteen US patents.
Based on analysis by DrugPatentWatch, the earliest date for a generic version of ERLEADA is ⤷ Try a Trial.
This potential generic entry date is based on patent ⤷ Try a Trial.
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
Patents protecting ERLEADA
Anti-androgens for the treatment of non-metastatic castrate-resistant prostate cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT IN COMBINATION WITH A GNRH AGONIST OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
Anti-androgens for the treatment of non-metastatic castrate-resistant prostate cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT IN COMBINATION WITH A GNRH AGONIST OF HIGH RISK NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
Anti-androgens for the treatment of non-metastatic castration-resistant prostate cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT IN COMBINATION WITH ANDROGEN DEPRIVATION THERAPY OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NMCRPC) THAT IMPROVES METASTASIS FREE SURVIVAL
Anti-androgens for the treatment of non-metastatic castrate-resistant prostate cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT IN COMBINATION WITH ORCHIECTOMY OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NMCRPC)
Androgen receptor modulator for the treatment of prostate cancer and androgen receptor-associated diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF METASTATIC CASTRATION-SENSITIVE PROSTATE CANCER (MCSPC)
Androgen receptor modulator for the treatment of prostate cancer and androgen receptor-associated diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
Androgen receptor modulator for the treatment of prostate cancer and androgen receptor-associated diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
Androgen receptor modulator for the treatment of prostate cancer and androgen receptor-associated diseases
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF METASTATIC CASTRATION-SENSITIVE PROSTATE CANCER (MCSPC)
Substituted diazaspiroalkanes as androgen receptor modulators
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Crystalline forms of an androgen receptor modulator
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF METASTATIC CASTRATION-SENSITIVE PROSTATE CANCER (MCSPC)
Crystalline forms of an androgen receptor modulator
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
Anti-androgens for the treatment of non-metastatic castrate-resistant prostate cancer
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
Substituted diazaspiroalkanes as androgen receptor modulators
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT IN COMBINATION WITH A GNRH AGONIST OF NON-METASTATIC, CASTRATION-RESISTANT PROSTATE CANCER (NM-CRPC)
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-001 | Feb 14, 2018 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-002 | Feb 17, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Janssen Biotech | ERLEADA | apalutamide | TABLET;ORAL | 210951-002 | Feb 17, 2023 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for ERLEADA
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Janssen-Cilag International NV | Erleada | apalutamide | EMEA/H/C/004452 Erleada is indicated:in adult men for the treatment of non metastatic castration resistant prostate cancer (nmCRPC) who are at high risk of developing metastatic disease.in adult men for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC) in combination with androgen deprivation therapy (ADT). |
Authorised | no | no | no | 2019-01-14 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for ERLEADA
When does loss-of-exclusivity occur for ERLEADA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Australia
Patent: 13271751
Estimated Expiration: ⤷ Try a Trial
Patent: 17200298
Estimated Expiration: ⤷ Try a Trial
Patent: 17279807
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2014030678
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 75767
Estimated Expiration: ⤷ Try a Trial
Patent: 08345
Estimated Expiration: ⤷ Try a Trial
Patent: 55660
Estimated Expiration: ⤷ Try a Trial
Patent: 14726
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 14003331
Estimated Expiration: ⤷ Try a Trial
China
Patent: 4619692
Estimated Expiration: ⤷ Try a Trial
Patent: 5693692
Estimated Expiration: ⤷ Try a Trial
Patent: 3135892
Estimated Expiration: ⤷ Try a Trial
Colombia
Patent: 40407
Estimated Expiration: ⤷ Try a Trial
Costa Rica
Patent: 140549
Estimated Expiration: ⤷ Try a Trial
Patent: 190331
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0180902
Estimated Expiration: ⤷ Try a Trial
Patent: 0201387
Estimated Expiration: ⤷ Try a Trial
Patent: 0210909
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 20393
Estimated Expiration: ⤷ Try a Trial
Patent: 23427
Estimated Expiration: ⤷ Try a Trial
Patent: 24831
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 58985
Estimated Expiration: ⤷ Try a Trial
Patent: 48553
Estimated Expiration: ⤷ Try a Trial
Patent: 33792
Estimated Expiration: ⤷ Try a Trial
Ecuador
Patent: 14030098
Estimated Expiration: ⤷ Try a Trial
Eurasian Patent Organization
Patent: 8791
Estimated Expiration: ⤷ Try a Trial
Patent: 3956
Estimated Expiration: ⤷ Try a Trial
Patent: 1492272
Estimated Expiration: ⤷ Try a Trial
Patent: 1791592
Estimated Expiration: ⤷ Try a Trial
Patent: 1992010
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 58985
Estimated Expiration: ⤷ Try a Trial
Patent: 48553
Estimated Expiration: ⤷ Try a Trial
Patent: 33792
Estimated Expiration: ⤷ Try a Trial
Patent: 22629
Estimated Expiration: ⤷ Try a Trial
France
Patent: C1050
Estimated Expiration: ⤷ Try a Trial
Guatemala
Patent: 1400283
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 10175
Estimated Expiration: ⤷ Try a Trial
Patent: 26066
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 38082
Estimated Expiration: ⤷ Try a Trial
Patent: 50357
Estimated Expiration: ⤷ Try a Trial
Patent: 54595
Estimated Expiration: ⤷ Try a Trial
Patent: 100047
Estimated Expiration: ⤷ Try a Trial
India
Patent: 084DEN2014
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 9738
Estimated Expiration: ⤷ Try a Trial
Patent: 7608
Estimated Expiration: ⤷ Try a Trial
Patent: 5413
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 82209
Estimated Expiration: ⤷ Try a Trial
Patent: 45821
Estimated Expiration: ⤷ Try a Trial
Patent: 15518890
Estimated Expiration: ⤷ Try a Trial
Patent: 17178923
Estimated Expiration: ⤷ Try a Trial
Patent: 18141009
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 2021525
Estimated Expiration: ⤷ Try a Trial
Patent: 58985
Estimated Expiration: ⤷ Try a Trial
Patent: 48553
Estimated Expiration: ⤷ Try a Trial
Patent: 33792
Estimated Expiration: ⤷ Try a Trial
Malaysia
Patent: 7500
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 6754
Estimated Expiration: ⤷ Try a Trial
Patent: 14015005
Estimated Expiration: ⤷ Try a Trial
Montenegro
Patent: 081
Estimated Expiration: ⤷ Try a Trial
Patent: 815
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 2203
Estimated Expiration: ⤷ Try a Trial
Patent: 7683
Estimated Expiration: ⤷ Try a Trial
Nicaragua
Patent: 1400142
Estimated Expiration: ⤷ Try a Trial
Norway
Patent: 21046
Estimated Expiration: ⤷ Try a Trial
Peru
Patent: 150631
Estimated Expiration: ⤷ Try a Trial
Patent: 200725
Estimated Expiration: ⤷ Try a Trial
Philippines
Patent: 014502714
Estimated Expiration: ⤷ Try a Trial
Patent: 016501470
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 58985
Estimated Expiration: ⤷ Try a Trial
Patent: 48553
Estimated Expiration: ⤷ Try a Trial
Patent: 33792
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 58985
Estimated Expiration: ⤷ Try a Trial
Patent: 48553
Estimated Expiration: ⤷ Try a Trial
Patent: 33792
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 370
Estimated Expiration: ⤷ Try a Trial
Patent: 617
Estimated Expiration: ⤷ Try a Trial
Patent: 988
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201610248S
Estimated Expiration: ⤷ Try a Trial
Patent: 201610249T
Estimated Expiration: ⤷ Try a Trial
Patent: 201408140Q
Estimated Expiration: ⤷ Try a Trial
Slovenia
Patent: 58985
Estimated Expiration: ⤷ Try a Trial
Patent: 48553
Estimated Expiration: ⤷ Try a Trial
Patent: 33792
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1500076
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2062024
Estimated Expiration: ⤷ Try a Trial
Patent: 2195916
Estimated Expiration: ⤷ Try a Trial
Patent: 150021993
Estimated Expiration: ⤷ Try a Trial
Patent: 190132543
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 70683
Estimated Expiration: ⤷ Try a Trial
Patent: 09738
Estimated Expiration: ⤷ Try a Trial
Patent: 75932
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 32732
Estimated Expiration: ⤷ Try a Trial
Patent: 1402561
Estimated Expiration: ⤷ Try a Trial
Turkey
Patent: 1808939
Estimated Expiration: ⤷ Try a Trial
Ukraine
Patent: 5665
Estimated Expiration: ⤷ Try a Trial
Patent: 3142
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering ERLEADA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Lithuania | 2656842 | ⤷ Try a Trial | |
| Japan | 6527621 | ⤷ Try a Trial | |
| Denmark | 3100727 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for ERLEADA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3533792 | 2021C/545 | Belgium | ⤷ Try a Trial | PRODUCT NAME: APALUTAMIDE; AUTHORISATION NUMBER AND DATE: EU/1/18/1342 20190116 |
| 3533792 | PA2021525 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: APALUTAMIDAS; REGISTRATION NO/DATE: EU/1/18/1342 20190114 |
| 2368550 | C20190027 00294 | Estonia | ⤷ Try a Trial | PRODUCT NAME: APALUTAMIID;REG NO/DATE: EU/1/18/1342 16.01.2019 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


