BONJESTA Drug Patent Profile
✉ Email this page to a colleague
When do Bonjesta patents expire, and when can generic versions of Bonjesta launch?
Bonjesta is a drug marketed by Duchesnay and is included in one NDA. There are four patents protecting this drug and one Paragraph IV challenge.
This drug has sixty-four patent family members in thirty-one countries.
The generic ingredient in BONJESTA is doxylamine succinate; pyridoxine hydrochloride. There are fourteen drug master file entries for this compound. Seven suppliers are listed for this compound. Additional details are available on the doxylamine succinate; pyridoxine hydrochloride profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Bonjesta
A generic version of BONJESTA was approved as doxylamine succinate; pyridoxine hydrochloride by ACTAVIS LABS FL INC on August 19th, 2016.
Summary for BONJESTA
| International Patents: | 64 |
| US Patents: | 4 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 1 |
| Clinical Trials: | 1 |
| Formulation / Manufacturing: | see details |
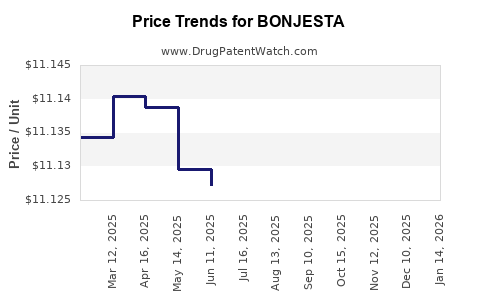
| Drug Prices: | Drug price information for BONJESTA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BONJESTA |
| What excipients (inactive ingredients) are in BONJESTA? | BONJESTA excipients list |
| DailyMed Link: | BONJESTA at DailyMed |
Recent Clinical Trials for BONJESTA
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Duchesnay Inc. | Phase 3 |
| Health Decisions | Phase 3 |
Pharmacology for BONJESTA
| Ingredient-type | Analogs/Derivatives Vitamin B 6 |
| Drug Class | Antihistamine Vitamin B6 Analog |
| Mechanism of Action | Histamine Receptor Antagonists |
Anatomical Therapeutic Chemical (ATC) Classes for BONJESTA
Paragraph IV (Patent) Challenges for BONJESTA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BONJESTA | Extended-release Tablets | doxylamine succinate; pyridoxine hydrochloride | 20 mg/20 mg | 209661 | 1 | 2018-08-28 |
US Patents and Regulatory Information for BONJESTA
BONJESTA is protected by four US patents.
Patents protecting BONJESTA
Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NAUSEA AND VOMITING OF PREGNANCY IN WOMEN WHO DO NOT RESPOND TO CONSERVATIVE MANAGEMENT
Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NAUSEA AND VOMITING OF PREGNANCY IN WOMEN WHO DO NOT RESPOND TO CONSERVATIVE MANAGEMENT
Plurimodal release formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NAUSEA AND VOMITING OF PREGNANCY IN WOMEN WHO DO NOT RESPOND TO CONSERVATIVE MANAGEMENT
Formulation of doxylamine and pyridoxine and/or metabolites or salts thereof
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF NAUSEA AND VOMITING OF PREGNANCY IN WOMEN WHO DO NOT RESPOND TO CONSERVATIVE MANAGEMENT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for BONJESTA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Duchesnay | BONJESTA | doxylamine succinate; pyridoxine hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 209661-001 | Nov 7, 2016 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for BONJESTA
When does loss-of-exclusivity occur for BONJESTA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 0126
Estimated Expiration: ⤷ Try a Trial
Patent: 2580
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 13224598
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2014020186
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 48798
Estimated Expiration: ⤷ Try a Trial
Chile
Patent: 14001828
Estimated Expiration: ⤷ Try a Trial
China
Patent: 4136004
Estimated Expiration: ⤷ Try a Trial
Cyprus
Patent: 23122
Estimated Expiration: ⤷ Try a Trial
Denmark
Patent: 26611
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 87971
Estimated Expiration: ⤷ Try a Trial
Patent: 26611
Estimated Expiration: ⤷ Try a Trial
Hong Kong
Patent: 97035
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 52301
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 3644
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 14701
Estimated Expiration: ⤷ Try a Trial
Patent: 15508082
Estimated Expiration: ⤷ Try a Trial
Patent: 16053092
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 26611
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 5912
Estimated Expiration: ⤷ Try a Trial
Patent: 14008594
Estimated Expiration: ⤷ Try a Trial
New Zealand
Patent: 7593
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 26611
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 26611
Estimated Expiration: ⤷ Try a Trial
Singapore
Patent: 201403931Y
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 1588259
Estimated Expiration: ⤷ Try a Trial
Patent: 140139496
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 09713
Estimated Expiration: ⤷ Try a Trial
Taiwan
Patent: 38657
Estimated Expiration: ⤷ Try a Trial
Patent: 1334780
Estimated Expiration: ⤷ Try a Trial
Uruguay
Patent: 631
Estimated Expiration: ⤷ Try a Trial
Patent: 283
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BONJESTA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Malaysia | 196073 | PLURIMODAL RELEASE FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF | ⤷ Try a Trial |
| Turkey | 201809565 | ⤷ Try a Trial | |
| Taiwan | 201613584 | Plurimodal release formulation of doxylamine and pyridoxine and/or metabolites or salts thereof | ⤷ Try a Trial |
| South Africa | 201701063 | PLURIMODAL RELEASE FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF | ⤷ Try a Trial |
| Canada | 2848798 | FORMULATION DE DOXYLAMINE ET DE PYRIDOXINE ET/OU DE LEURS METABOLITES OU SELS (FORMULATION OF DOXYLAMINE AND PYRIDOXINE AND/OR METABOLITES OR SALTS THEREOF) | ⤷ Try a Trial |
| Netherlands | 1023797 | Farmaceutische doseringsvorm die zwangerschapsvriendelijke onderscheidingstekens draagt. | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


