BIJUVA Drug Patent Profile
✉ Email this page to a colleague
Which patents cover Bijuva, and when can generic versions of Bijuva launch?
Bijuva is a drug marketed by Mayne Pharma and is included in one NDA. There are twenty-four patents protecting this drug and one Paragraph IV challenge.
This drug has one hundred and sixty-nine patent family members in twenty-one countries.
The generic ingredient in BIJUVA is estradiol; progesterone. There are seventy-five drug master file entries for this compound. Three suppliers are listed for this compound. Additional details are available on the estradiol; progesterone profile page.
DrugPatentWatch® Generic Entry Outlook for Bijuva
There have been two patent litigation cases involving the patents protecting this drug, indicating strong interest in generic launch. Recent data indicate that 63% of patent challenges are decided in favor of the generic patent challenger and that 54% of successful patent challengers promptly launch generic drugs.
Indicators of Generic Entry
Summary for BIJUVA
| International Patents: | 169 |
| US Patents: | 24 |
| Applicants: | 1 |
| NDAs: | 1 |
| Finished Product Suppliers / Packagers: | 2 |
| Formulation / Manufacturing: | see details |
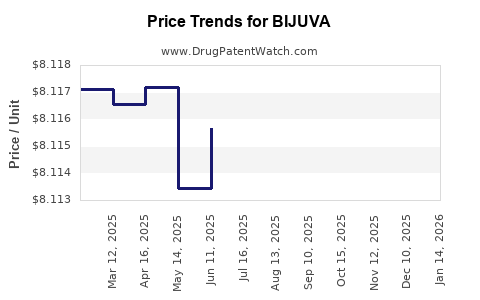
| Drug Prices: | Drug price information for BIJUVA |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for BIJUVA |
| What excipients (inactive ingredients) are in BIJUVA? | BIJUVA excipients list |
| DailyMed Link: | BIJUVA at DailyMed |
Pharmacology for BIJUVA
| Drug Class | Estrogen Progesterone |
| Mechanism of Action | Estrogen Receptor Agonists |
Anatomical Therapeutic Chemical (ATC) Classes for BIJUVA
Paragraph IV (Patent) Challenges for BIJUVA
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BIJUVA | Capsules | estradiol; progesterone | 1 mg/100 mg | 210132 | 1 | 2020-01-06 |
US Patents and Regulatory Information for BIJUVA
BIJUVA is protected by twenty-four US patents.
Patents protecting BIJUVA
Progesterone formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Progesterone formulations
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Progesterone formulations having a desirable pk profile
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
Natural combination hormone replacement formulations and therapies
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF MENOPAUSE SYMPTOMS, INCLUDING VASOMOTOR SYMPTOMS
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mayne Pharma | BIJUVA | estradiol; progesterone | CAPSULE;ORAL | 210132-001 | Oct 28, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mayne Pharma | BIJUVA | estradiol; progesterone | CAPSULE;ORAL | 210132-001 | Oct 28, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mayne Pharma | BIJUVA | estradiol; progesterone | CAPSULE;ORAL | 210132-001 | Oct 28, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mayne Pharma | BIJUVA | estradiol; progesterone | CAPSULE;ORAL | 210132-001 | Oct 28, 2018 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for BIJUVA
When does loss-of-exclusivity occur for BIJUVA?
Based on analysis by DrugPatentWatch, the following patents block generic entry in the countries listed below:
Argentina
Patent: 5619
Estimated Expiration: ⤷ Try a Trial
Patent: 8160
Estimated Expiration: ⤷ Try a Trial
Patent: 9872
Estimated Expiration: ⤷ Try a Trial
Patent: 7022
Estimated Expiration: ⤷ Try a Trial
Australia
Patent: 6507
Estimated Expiration: ⤷ Try a Trial
Patent: 12340589
Estimated Expiration: ⤷ Try a Trial
Patent: 13211876
Estimated Expiration: ⤷ Try a Trial
Patent: 13277233
Estimated Expiration: ⤷ Try a Trial
Patent: 13277234
Estimated Expiration: ⤷ Try a Trial
Patent: 13277235
Estimated Expiration: ⤷ Try a Trial
Patent: 13277236
Estimated Expiration: ⤷ Try a Trial
Patent: 14349132
Estimated Expiration: ⤷ Try a Trial
Patent: 15237243
Estimated Expiration: ⤷ Try a Trial
Patent: 16366200
Estimated Expiration: ⤷ Try a Trial
Patent: 17206262
Estimated Expiration: ⤷ Try a Trial
Patent: 17208300
Estimated Expiration: ⤷ Try a Trial
Patent: 17394679
Estimated Expiration: ⤷ Try a Trial
Patent: 18222947
Estimated Expiration: ⤷ Try a Trial
Patent: 18280270
Estimated Expiration: ⤷ Try a Trial
Patent: 19204653
Estimated Expiration: ⤷ Try a Trial
Patent: 19204655
Estimated Expiration: ⤷ Try a Trial
Patent: 19204658
Estimated Expiration: ⤷ Try a Trial
Patent: 21218231
Estimated Expiration: ⤷ Try a Trial
Patent: 21240253
Estimated Expiration: ⤷ Try a Trial
Brazil
Patent: 2014012444
Estimated Expiration: ⤷ Try a Trial
Patent: 2014018439
Estimated Expiration: ⤷ Try a Trial
Patent: 2014031824
Estimated Expiration: ⤷ Try a Trial
Patent: 2014031837
Estimated Expiration: ⤷ Try a Trial
Patent: 2014031910
Estimated Expiration: ⤷ Try a Trial
Patent: 2014031914
Estimated Expiration: ⤷ Try a Trial
Patent: 2016009008
Estimated Expiration: ⤷ Try a Trial
Patent: 2018011483
Estimated Expiration: ⤷ Try a Trial
Patent: 2019011655
Estimated Expiration: ⤷ Try a Trial
Patent: 2019025914
Estimated Expiration: ⤷ Try a Trial
Canada
Patent: 6044
Estimated Expiration: ⤷ Try a Trial
Patent: 56520
Estimated Expiration: ⤷ Try a Trial
Patent: 61346
Estimated Expiration: ⤷ Try a Trial
Patent: 76947
Estimated Expiration: ⤷ Try a Trial
Patent: 76964
Estimated Expiration: ⤷ Try a Trial
Patent: 76968
Estimated Expiration: ⤷ Try a Trial
Patent: 76977
Estimated Expiration: ⤷ Try a Trial
Patent: 26342
Estimated Expiration: ⤷ Try a Trial
Patent: 42568
Estimated Expiration: ⤷ Try a Trial
Patent: 07636
Estimated Expiration: ⤷ Try a Trial
Patent: 45024
Estimated Expiration: ⤷ Try a Trial
China
Patent: 0290793
Estimated Expiration: ⤷ Try a Trial
Croatia
Patent: 0210861
Estimated Expiration: ⤷ Try a Trial
Patent: 0211377
Estimated Expiration: ⤷ Try a Trial
European Patent Office
Patent: 82584
Estimated Expiration: ⤷ Try a Trial
Patent: 06742
Estimated Expiration: ⤷ Try a Trial
Patent: 61072
Estimated Expiration: ⤷ Try a Trial
Patent: 61073
Estimated Expiration: ⤷ Try a Trial
Patent: 61233
Estimated Expiration: ⤷ Try a Trial
Patent: 61234
Estimated Expiration: ⤷ Try a Trial
Patent: 60179
Estimated Expiration: ⤷ Try a Trial
Patent: 22364
Estimated Expiration: ⤷ Try a Trial
Patent: 86514
Estimated Expiration: ⤷ Try a Trial
Patent: 48036
Estimated Expiration: ⤷ Try a Trial
Patent: 60500
Estimated Expiration: ⤷ Try a Trial
Patent: 09586
Estimated Expiration: ⤷ Try a Trial
Patent: 36133
Estimated Expiration: ⤷ Try a Trial
Patent: 09646
Estimated Expiration: ⤷ Try a Trial
France
Patent: C1058
Estimated Expiration: ⤷ Try a Trial
Hungary
Patent: 55275
Estimated Expiration: ⤷ Try a Trial
Patent: 55562
Estimated Expiration: ⤷ Try a Trial
Israel
Patent: 6358
Estimated Expiration: ⤷ Try a Trial
Patent: 6359
Estimated Expiration: ⤷ Try a Trial
Patent: 5139
Estimated Expiration: ⤷ Try a Trial
Patent: 9884
Estimated Expiration: ⤷ Try a Trial
Patent: 7023
Estimated Expiration: ⤷ Try a Trial
Japan
Patent: 24393
Estimated Expiration: ⤷ Try a Trial
Patent: 85866
Estimated Expiration: ⤷ Try a Trial
Patent: 98460
Estimated Expiration: ⤷ Try a Trial
Patent: 34519
Estimated Expiration: ⤷ Try a Trial
Patent: 42334
Estimated Expiration: ⤷ Try a Trial
Patent: 42389
Estimated Expiration: ⤷ Try a Trial
Patent: 97402
Estimated Expiration: ⤷ Try a Trial
Patent: 56215
Estimated Expiration: ⤷ Try a Trial
Patent: 80672
Estimated Expiration: ⤷ Try a Trial
Patent: 82127
Estimated Expiration: ⤷ Try a Trial
Patent: 98177
Estimated Expiration: ⤷ Try a Trial
Patent: 15504924
Estimated Expiration: ⤷ Try a Trial
Patent: 15507607
Estimated Expiration: ⤷ Try a Trial
Patent: 15519405
Estimated Expiration: ⤷ Try a Trial
Patent: 15520235
Estimated Expiration: ⤷ Try a Trial
Patent: 15520236
Estimated Expiration: ⤷ Try a Trial
Patent: 15520237
Estimated Expiration: ⤷ Try a Trial
Patent: 16534025
Estimated Expiration: ⤷ Try a Trial
Patent: 17509630
Estimated Expiration: ⤷ Try a Trial
Patent: 18024685
Estimated Expiration: ⤷ Try a Trial
Patent: 18024688
Estimated Expiration: ⤷ Try a Trial
Patent: 18199711
Estimated Expiration: ⤷ Try a Trial
Patent: 18538290
Estimated Expiration: ⤷ Try a Trial
Patent: 19206540
Estimated Expiration: ⤷ Try a Trial
Patent: 19214598
Estimated Expiration: ⤷ Try a Trial
Patent: 20100642
Estimated Expiration: ⤷ Try a Trial
Patent: 20504093
Estimated Expiration: ⤷ Try a Trial
Patent: 21119155
Estimated Expiration: ⤷ Try a Trial
Lithuania
Patent: 82584
Estimated Expiration: ⤷ Try a Trial
Mexico
Patent: 8435
Estimated Expiration: ⤷ Try a Trial
Patent: 5818
Estimated Expiration: ⤷ Try a Trial
Patent: 7596
Estimated Expiration: ⤷ Try a Trial
Patent: 14006256
Estimated Expiration: ⤷ Try a Trial
Patent: 14009093
Estimated Expiration: ⤷ Try a Trial
Patent: 14015897
Estimated Expiration: ⤷ Try a Trial
Patent: 14015898
Estimated Expiration: ⤷ Try a Trial
Patent: 14015899
Estimated Expiration: ⤷ Try a Trial
Patent: 14015900
Estimated Expiration: ⤷ Try a Trial
Patent: 16005092
Estimated Expiration: ⤷ Try a Trial
Patent: 16011706
Estimated Expiration: ⤷ Try a Trial
Patent: 18006882
Estimated Expiration: ⤷ Try a Trial
Patent: 19006513
Estimated Expiration: ⤷ Try a Trial
Patent: 20013533
Estimated Expiration: ⤷ Try a Trial
Patent: 22002614
Estimated Expiration: ⤷ Try a Trial
Poland
Patent: 82584
Estimated Expiration: ⤷ Try a Trial
Patent: 61073
Estimated Expiration: ⤷ Try a Trial
Portugal
Patent: 82584
Estimated Expiration: ⤷ Try a Trial
Russian Federation
Patent: 13888
Estimated Expiration: ⤷ Try a Trial
Patent: 40059
Estimated Expiration: ⤷ Try a Trial
Patent: 15100531
Estimated Expiration: ⤷ Try a Trial
Patent: 15100533
Estimated Expiration: ⤷ Try a Trial
Patent: 16118396
Estimated Expiration: ⤷ Try a Trial
Patent: 16136666
Estimated Expiration: ⤷ Try a Trial
Patent: 19115913
Estimated Expiration: ⤷ Try a Trial
Patent: 19139675
Estimated Expiration: ⤷ Try a Trial
Patent: 19142696
Estimated Expiration: ⤷ Try a Trial
Patent: 20140867
Estimated Expiration: ⤷ Try a Trial
Serbia
Patent: 297
Estimated Expiration: ⤷ Try a Trial
South Africa
Patent: 1500212
Estimated Expiration: ⤷ Try a Trial
South Korea
Patent: 2163369
Estimated Expiration: ⤷ Try a Trial
Patent: 2177782
Estimated Expiration: ⤷ Try a Trial
Patent: 2335160
Estimated Expiration: ⤷ Try a Trial
Patent: 2488424
Estimated Expiration: ⤷ Try a Trial
Patent: 150028302
Estimated Expiration: ⤷ Try a Trial
Patent: 150032560
Estimated Expiration: ⤷ Try a Trial
Patent: 160062097
Estimated Expiration: ⤷ Try a Trial
Patent: 160137597
Estimated Expiration: ⤷ Try a Trial
Patent: 180100567
Estimated Expiration: ⤷ Try a Trial
Patent: 200013771
Estimated Expiration: ⤷ Try a Trial
Patent: 200018383
Estimated Expiration: ⤷ Try a Trial
Patent: 200128214
Estimated Expiration: ⤷ Try a Trial
Patent: 210107915
Estimated Expiration: ⤷ Try a Trial
Patent: 210148435
Estimated Expiration: ⤷ Try a Trial
Patent: 220080205
Estimated Expiration: ⤷ Try a Trial
Patent: 230021170
Estimated Expiration: ⤷ Try a Trial
Spain
Patent: 69250
Estimated Expiration: ⤷ Try a Trial
Patent: 85523
Estimated Expiration: ⤷ Try a Trial
Generics may enter earlier, or later, based on new patent filings, patent extensions, patent invalidation, early generic licensing, generic entry preferences, and other factors.
See the table below for additional patents covering BIJUVA around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Japan | 6656215 | ⤷ Try a Trial | |
| Russian Federation | 2015100531 | КАПСУЛЫ С РАСТВОРИМЫМ ЭСТРАДИОЛОМ ДЛЯ ИНТРАВАГИНАЛЬНОГО ВВЕДЕНИЯ | ⤷ Try a Trial |
| Japan | 2015520236 | 経皮的ホルモン補充療法 | ⤷ Try a Trial |
| South Korea | 102163369 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for BIJUVA
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2782584 | 21C1058 | France | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), Y COMPRIS SOUS FORME HEMIHYDRATEE, ET DE LA PROGESTERONE; NAT. REGISTRATION NO/DATE: NL51886 20210421; FIRST REGISTRATION: BE - BE582231 20210406 |
| 1380301 | 2009C/007 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE-ETHINYLESTRADIOL; AUTHORISATION NUMBER AND DATE: BE321386 20080811 |
| 2782584 | 301153 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTAINING BOTH ESTRADIOL (17SS-ESTRADIOL), OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, HYDRATE OR SOLVATE THEREOF (INCLUDING IN HEMIHYDRATE FORM), AND PROGESTERONE; NATIONAL REGISTRATION NO/DATE: RVG 125821 20210611; FIRST REGISTRATION: BE BE582231 20210406 |
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


