ATORVASTATIN Drug Patent Profile
✉ Email this page to a colleague
When do Atorvastatin patents expire, and what generic alternatives are available?
Atorvastatin is a drug marketed by Accord Hlthcare, Alkem Labs Ltd, Apotex, Apotex Inc, Aurobindo Pharma Ltd, Biocon Pharma, Cadila Pharms Ltd, Chartwell Rx, Dr Reddys, Dr Reddys Labs Ltd, Graviti Pharms, Hetero Labs Ltd V, Invagen Pharms, Lannett Co Inc, Laurus, Lepu Pharm, Lupin Ltd, Macleods Pharms Ltd, Mankind Pharma, Micro Labs Ltd India, MSN, Mylan Pharms Inc, Perrigo R And D, Sciegen Pharms Inc, Shandong Xinhua, Strides Pharma, Sun Pharm Inds Ltd, Teva Pharms, Teva Pharms Inc, Teva Pharms Usa, Umedica, and Zydus Pharms. and is included in thirty-three NDAs.
The generic ingredient in ATORVASTATIN is atorvastatin calcium. There are sixty-two drug master file entries for this compound. Sixty-one suppliers are listed for this compound. Additional details are available on the atorvastatin calcium profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Atorvastatin
A generic version of ATORVASTATIN was approved as atorvastatin calcium by APOTEX INC on May 29th, 2012.
AI Deep Research
Questions you can ask:
- What is the 5 year forecast for ATORVASTATIN?
- What are the global sales for ATORVASTATIN?
- What is Average Wholesale Price for ATORVASTATIN?
Summary for ATORVASTATIN
| US Patents: | 0 |
| Applicants: | 32 |
| NDAs: | 33 |
| Drug Prices: | Drug price information for ATORVASTATIN |
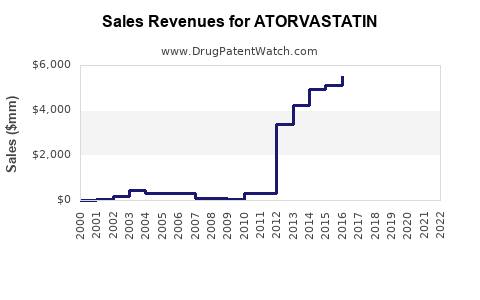
| Drug Sales Revenues: | Drug sales revenues for ATORVASTATIN |
| DailyMed Link: | ATORVASTATIN at DailyMed |
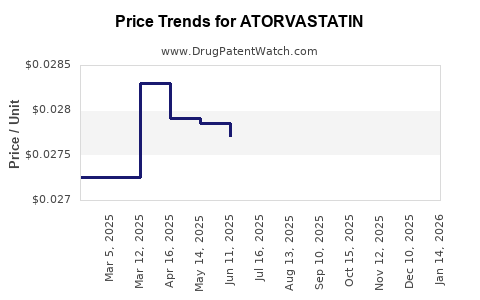
See drug prices for ATORVASTATIN