AFINITOR Drug Patent Profile
✉ Email this page to a colleague
When do Afinitor patents expire, and what generic alternatives are available?
Afinitor is a drug marketed by Novartis and Novartis Pharm and is included in two NDAs. There are two patents protecting this drug and two Paragraph IV challenges.
This drug has two hundred and twenty-nine patent family members in thirty-one countries.
The generic ingredient in AFINITOR is everolimus. There are twelve drug master file entries for this compound. Nine suppliers are listed for this compound. Additional details are available on the everolimus profile page.
DrugPatentWatch® Litigation and Generic Entry Outlook for Afinitor
A generic version of AFINITOR was approved as everolimus by HIKMA on April 12th, 2018.
Summary for AFINITOR
| International Patents: | 229 |
| US Patents: | 2 |
| Applicants: | 2 |
| NDAs: | 2 |
| Finished Product Suppliers / Packagers: | 1 |
| Raw Ingredient (Bulk) Api Vendors: | 38 |
| Clinical Trials: | 180 |
| Patent Applications: | 5,223 |
| Formulation / Manufacturing: | see details |
| Drug Prices: | Drug price information for AFINITOR |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for AFINITOR |
| What excipients (inactive ingredients) are in AFINITOR? | AFINITOR excipients list |
| DailyMed Link: | AFINITOR at DailyMed |

Recent Clinical Trials for AFINITOR
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Nationwide Children's Hospital | Phase 2 |
| Stemline Therapeutics, Inc. | Phase 1/Phase 2 |
| Peking University | Phase 2 |
Pharmacology for AFINITOR
| Drug Class | Kinase Inhibitor mTOR Inhibitor Immunosuppressant |
| Mechanism of Action | Cytochrome P450 2D6 Inhibitors Cytochrome P450 3A4 Inhibitors Protein Kinase Inhibitors mTOR Inhibitors |
| Physiological Effect | Decreased Immunologic Activity |
Anatomical Therapeutic Chemical (ATC) Classes for AFINITOR
Paragraph IV (Patent) Challenges for AFINITOR
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| AFINITOR | Tablets | everolimus | 2.5 mg, 5 mg, and 7.5 mg | 022334 | 1 | 2014-12-10 |
| AFINITOR | Tablets | everolimus | 10 mg | 022334 | 1 | 2014-06-18 |
US Patents and Regulatory Information for AFINITOR
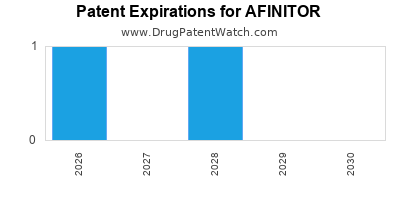
AFINITOR is protected by one US patents.
Patents protecting AFINITOR
Neuroendocrine tumor treatment
Patent Number: ⤷ Try a Trial
Patent Expiration: ⤷ Try a Trial
Patented Use: TREATMENT OF PATIENTS WITH PROGRESSIVE NEUROENDOCRINE TUMORS OF PANCREATIC ORIGIN (PNET) THAT ARE UNRESECTABLE, LOCALLY ADVANCED OR METASTATIC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-003 | Jul 9, 2010 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-002 | Mar 30, 2009 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-001 | Mar 30, 2009 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-002 | Mar 30, 2009 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-003 | Jul 9, 2010 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-001 | Mar 30, 2009 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for AFINITOR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-002 | Mar 30, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-003 | Jul 9, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-004 | Mar 30, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-001 | Mar 30, 2009 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-003 | Jul 9, 2010 | ⤷ Try a Trial | ⤷ Try a Trial |
| Novartis | AFINITOR | everolimus | TABLET;ORAL | 022334-004 | Mar 30, 2012 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for AFINITOR
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Novartis Europharm Limited | Afinitor | everolimus | EMEA/H/C/001038 Hormone-receptor-positive advanced breast cancerAfinitor is indicated for the treatment of hormone-receptor-positive, HER2/neu-negative advanced breast cancer, in combination with exemestane, in post-menopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor.Neuroendocrine tumours of pancreatic originAfinitor is indicated for the treatment of unresectable or metastatic, well or moderately differentiated neuroendocrine tumours of pancreatic origin in adults with progressive disease.Neuroendocrine tumours of gastrointestinal or lung originAfinitor is indicated for the treatment of unresectable or metastatic, well-differentiated (Grade 1 or Grade 2) non-functional neuroendocrine tumours of gastrointestinal or lung origin in adults with progressive disease.Renal-cell carcinomaAfinitor is indicated for the treatment of patients with advanced renal-cell carcinoma, whose disease has progressed on or after treatment with VEGF-targeted therapy. |
Authorised | no | no | no | 2009-08-02 | |
| Novartis Europharm Limited | Votubia | everolimus | EMEA/H/C/002311 Renal angiomyolipoma associated with tuberous sclerosis complex (TSC)Votubia is indicated for the treatment of adult patients with renal angiomyolipoma associated with tuberous sclerosis complex (TSC) who are at risk of complications (based on factors such as tumour size or presence of aneurysm, or presence of multiple or bilateral tumours) but who do not require immediate surgery.The evidence is based on analysis of change in sum of angiomyolipoma volume.Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC)Votubia is indicated for the treatment of patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not amenable to surgery.The evidence is based on analysis of change in SEGA volume. Further clinical benefit, such as improvement in disease‑related symptoms, has not been demonstrated. |
Authorised | no | no | no | 2011-09-02 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for AFINITOR
See the table below for patents covering AFINITOR around the world.
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| European Patent Office | 2783686 | Association d'un dérivé de rapamycine et de létrozole pour le traitement du cancer du sein (Combination of a rapamycin derivative and letrozole for treating breast cancer) | ⤷ Try a Trial |
| China | 101360496 | Neuroendocrine tumor treatment using mTOR inhibitors | ⤷ Try a Trial |
| Norway | 2017067 | ⤷ Try a Trial | |
| Malaysia | 127579 | STABILIZATION OF MACROLIDES | ⤷ Try a Trial |
| Lithuania | 3351246 | ⤷ Try a Trial | |
| Poland | 208854 | ⤷ Try a Trial | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for AFINITOR
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 3342411 | LUC00148 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: EVEROLIMUS; AUTHORISATION NUMBER AND DATE: EU/1/09/538/001-008 20110830 |
| 2269604 | 93320 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: EVEROLIMUS OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI; AUTHORISATION NUMBER AND DATE: EU/1/09/538-001-006 - AFINITOR - EVEROLIMUS |
| 2269604 | 300849 | Netherlands | ⤷ Try a Trial | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), MERGE |
| 2269603 | CA 2015 00058 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EVEROLIMUS ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/09/538/001-008 (K(2012)5347) 20120725 |
| 2269604 | PA2016035,C2269604 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: EVEROLIMUZAS; REGISTRATION NO/DATE: EU/1/09/538/001-006 20090803 |
| 0663916 | C00663916/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: EVEROLIMUS; REGISTRATION NUMBER/DATE: SWISSMEDIC 56238 21.04.2005 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.


