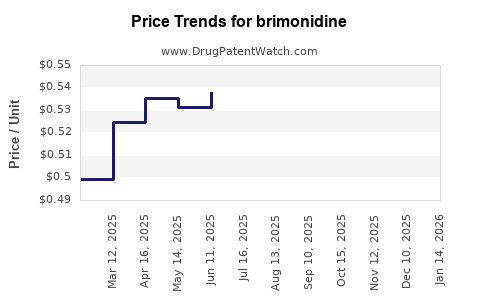
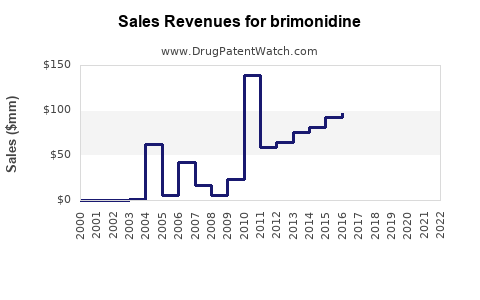
Brimonidine - Generic Drug Details
✉ Email this page to a colleague
Generic filers with tentative approvals for BRIMONIDINE
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | 0.2%; 0.5% | SOLUTION; OPHTHALMIC |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
US Patents and Regulatory Information for brimonidine
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbvie | COMBIGAN | brimonidine tartrate; timolol maleate | SOLUTION/DROPS;OPHTHALMIC | 021398-001 | Oct 30, 2007 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Bausch And Lomb | BRIMONIDINE TARTRATE | brimonidine tartrate | SOLUTION/DROPS;OPHTHALMIC | 076260-001 | May 28, 2003 | AT | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Alembic | BRIMONIDINE TARTRATE | brimonidine tartrate | SOLUTION/DROPS;OPHTHALMIC | 216909-001 | Aug 1, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |