Urea - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for urea and what is the scope of patent protection?
Urea
is the generic ingredient in eight branded drugs marketed by Hospira, Otsuka America, Metabolic Solutions, Dxs Devices, and Avent, and is included in six NDAs. Additional information is available in the individual branded drug profile pages.There are thirty-three drug master file entries for urea.
Summary for urea
| US Patents: | 0 |
| Tradenames: | 8 |
| Applicants: | 5 |
| NDAs: | 6 |
| Drug Master File Entries: | 33 |
| Raw Ingredient (Bulk) Api Vendors: | 216 |
| Patent Applications: | 6,220 |
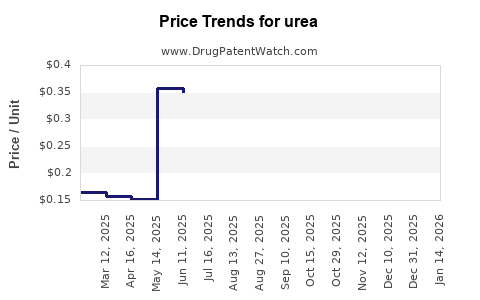
| Drug Prices: | Drug price trends for urea |
| DailyMed Link: | urea at DailyMed |
US Patents and Regulatory Information for urea
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avent | PYTEST KIT | urea, c-14 | CAPSULE;ORAL | 020617-002 | May 9, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Hospira | STERILE UREA | urea | INJECTABLE;INJECTION | 017698-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Otsuka America | MERETEK UBT KIT (W/ PRANACTIN) | urea c-13 | FOR SOLUTION;ORAL | 020586-001 | Sep 17, 1996 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |