Triamcinolone - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for triamcinolone and what is the scope of freedom to operate?
Triamcinolone
is the generic ingredient in thirty-one branded drugs marketed by Astellas, Delcor Asset Corp, Barr, Impax Labs, Ivax Sub Teva Pharms, Mylan, Purepac Pharm, Roxane, Sandoz, Teva, Watson Labs, Abbvie, Chattem Sanofi, Ivax Pharms, Solvay, Actavis Mid Atlantic, Alkem Labs Ltd, Alpharma Us Pharms, Ambix, Chartwell Rx, Cosette, Encube, Fougera Pharms, Glenmark Pharms Ltd, Macleods Pharms Ltd, Micro Labs, Morton Grove, Padagis Us, Pharmaderm, Pharmafair, Strides Pharma, Taro, Topiderm, Crown Labs, Savage Labs, Pacira Pharms Inc, Apothecon, Amneal, Eugia Pharma, Long Grove Pharms, Mylan Labs Ltd, Parnell, Teva Pharms Usa, Allergan, Harrow Eye, Epic Pharma Llc, Pai Holdings Pharm, Quagen, Wockhardt Bio Ag, Aurobindo Pharma Ltd, Cintex Svcs, Glenmark Pharms, Padagis Israel, Cmp Pharma Inc, Akorn, Lyne, Lupin Atlantis, Sanofi Aventis Us, Apotex, Perrigo Pharma Intl, Sun Pharm Inds Inc, Rising, Saptalis Pharms, Bausch And Lomb Inc, and Fosun Pharma, and is included in one hundred and sixty-seven NDAs. There are seven patents protecting this compound. Additional information is available in the individual branded drug profile pages.There are fifty-one drug master file entries for triamcinolone.
Summary for triamcinolone
| US Patents: | 7 |
| Tradenames: | 31 |
| Applicants: | 65 |
| NDAs: | 167 |
| Drug Master File Entries: | 51 |
| Raw Ingredient (Bulk) Api Vendors: | 76 |
| Clinical Trials: | 397 |
| Patent Applications: | 6,787 |
| Formulation / Manufacturing: | see details |
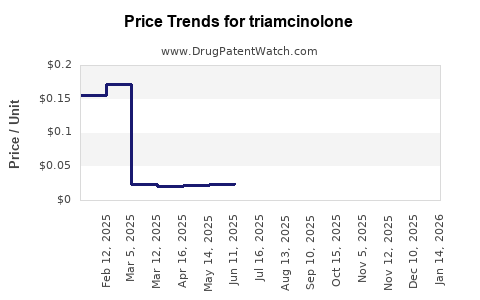
| Drug Prices: | Drug price trends for triamcinolone |
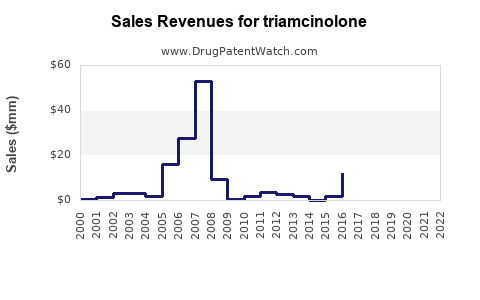
| Drug Sales Revenues: | Drug sales revenues for triamcinolone |
| DailyMed Link: | triamcinolone at DailyMed |
Recent Clinical Trials for triamcinolone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Centre hospitalier de l'Université de Montréal (CHUM) | N/A |
| Gadjah Mada University | Phase 1/Phase 2 |
| Centre for Interdisciplinary Research in Rehabilitation of Greater Montreal | Phase 4 |
Medical Subject Heading (MeSH) Categories for triamcinolone
US Patents and Regulatory Information for triamcinolone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astellas | ARISTOCORT A | triamcinolone acetonide | CREAM;TOPICAL | 083017-004 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Savage Labs | TRYMEX | triamcinolone acetonide | CREAM;TOPICAL | 088197-001 | Mar 25, 1983 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pharmaderm | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | CREAM;TOPICAL | 087990-001 | Jul 7, 1983 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Allergan | TRIVARIS | triamcinolone acetonide | INJECTABLE;INTRA-ARTICULAR, INTRAMUSCULAR, INTRAVITREAL | 022220-001 | Jun 16, 2008 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Padagis Us | TRIAMCINOLONE ACETONIDE | triamcinolone acetonide | OINTMENT;TOPICAL | 087385-001 | Approved Prior to Jan 1, 1982 | AT | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Delcor Asset Corp | KENALOG | triamcinolone acetonide | LOTION;TOPICAL | 084343-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Watson Labs | TRIAMCINOLONE DIACETATE | triamcinolone diacetate | INJECTABLE;INJECTION | 085529-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |