Sulfamethoxazole - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for sulfamethoxazole and what is the scope of patent protection?
Sulfamethoxazole
is the generic ingredient in twenty-six branded drugs marketed by Roche, Ascot, Barr, Heather, Rising, Watson Labs, Shionogi, Sun Pharm Inds Inc, Monarch Pharms, Abraxis Pharm, Bedford, Hikma, Hospira, Mylan Labs Ltd, Somerset, Teva Pharms Usa, Sun Pharm Industries, Ani Pharms, Aurobindo Pharma, Chartwell Molecular, Lupin Ltd, Novitium Pharma, Prasco, Teva, Pharm Assoc, Usl Pharma, Alpharma Us Pharms, Naska, Novel Labs Inc, Amneal Pharms Ny, Chartwell Molecules, Fosun Pharma, Glenmark Generics, Interpharm, Martec Usa Llc, Mutual Pharm, Pliva, Roxane, Vista Pharms, Heritage Pharma Avet, and Superpharm, and is included in seventy-five NDAs. Additional information is available in the individual branded drug profile pages.There are twenty-seven drug master file entries for sulfamethoxazole.
Summary for sulfamethoxazole
| US Patents: | 0 |
| Tradenames: | 26 |
| Applicants: | 41 |
| NDAs: | 75 |
| Drug Master File Entries: | 27 |
| Raw Ingredient (Bulk) Api Vendors: | 161 |
| Clinical Trials: | 157 |
| Patent Applications: | 6,870 |
| Formulation / Manufacturing: | see details |
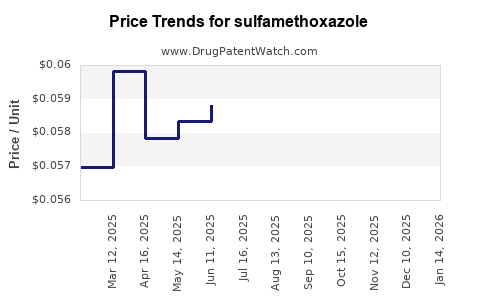
| Drug Prices: | Drug price trends for sulfamethoxazole |
| DailyMed Link: | sulfamethoxazole at DailyMed |
Recent Clinical Trials for sulfamethoxazole
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Johns Hopkins University | Early Phase 1 |
| The Cleveland Clinic | Phase 4 |
| Assistance Publique - Hôpitaux de Paris | Phase 4 |
Medical Subject Heading (MeSH) Categories for sulfamethoxazole
US Patents and Regulatory Information for sulfamethoxazole
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva | COTRIM D.S. | sulfamethoxazole; trimethoprim | TABLET;ORAL | 070048-001 | Mar 18, 1985 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Industries | BACTRIM | sulfamethoxazole; trimethoprim | SUSPENSION;ORAL | 017560-001 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mylan Labs Ltd | SULFAMETHOXAZOLE AND TRIMETHOPRIM | sulfamethoxazole; trimethoprim | INJECTABLE;INJECTION | 206607-001 | Aug 30, 2017 | AP | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |