Rufinamide - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for rufinamide and what is the scope of patent protection?
Rufinamide
is the generic ingredient in two branded drugs marketed by Eisai Inc, Alkem Labs Ltd, Aurobindo Pharma, Bionpharma, Chartwell Rx, Hetero Labs Ltd Iii, Hikma, Lupin Ltd, Glenmark Pharms Ltd, Micro Labs, and Mylan, and is included in sixteen NDAs. Additional information is available in the individual branded drug profile pages.There are seven drug master file entries for rufinamide. Eleven suppliers are listed for this compound.
Summary for rufinamide
| US Patents: | 0 |
| Tradenames: | 2 |
| Applicants: | 11 |
| NDAs: | 16 |
| Drug Master File Entries: | 7 |
| Finished Product Suppliers / Packagers: | 11 |
| Raw Ingredient (Bulk) Api Vendors: | 122 |
| Clinical Trials: | 12 |
| Patent Applications: | 1,609 |
| Formulation / Manufacturing: | see details |
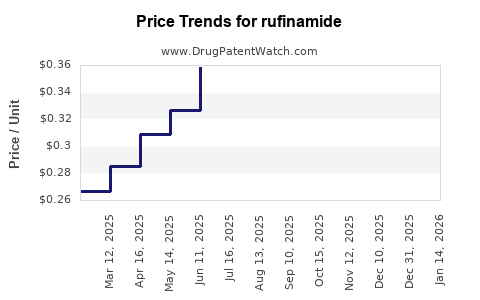
| Drug Prices: | Drug price trends for rufinamide |
| Patent Litigation and PTAB cases: | See patent lawsuits and PTAB cases for rufinamide |
| What excipients (inactive ingredients) are in rufinamide? | rufinamide excipients list |
| DailyMed Link: | rufinamide at DailyMed |
Recent Clinical Trials for rufinamide
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Guy's and St Thomas' NHS Foundation Trust | Phase 4 |
| The Leeds Teaching Hospitals NHS Trust | Phase 4 |
| Cambridge University Hospitals NHS Foundation Trust | Phase 4 |
Paragraph IV (Patent) Challenges for RUFINAMIDE
| Tradename | Dosage | Ingredient | Strength | NDA | ANDAs Submitted | Submissiondate |
|---|---|---|---|---|---|---|
| BANZEL | Oral Suspension | rufinamide | 40 mg/mL | 201367 | 1 | 2014-06-16 |
| BANZEL | Tablets | rufinamide | 200 mg and 400 mg | 021911 | 5 | 2012-11-14 |
US Patents and Regulatory Information for rufinamide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lupin Ltd | RUFINAMIDE | rufinamide | TABLET;ORAL | 204964-001 | Aug 17, 2022 | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-002 | Nov 14, 2008 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Glenmark Pharms Ltd | RUFINAMIDE | rufinamide | TABLET;ORAL | 205075-001 | May 16, 2016 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Lupin Ltd | RUFINAMIDE | rufinamide | SUSPENSION;ORAL | 213457-001 | Dec 18, 2020 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Aurobindo Pharma | RUFINAMIDE | rufinamide | TABLET;ORAL | 217230-002 | Jun 16, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-001 | Nov 14, 2008 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for rufinamide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-003 | Nov 14, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-002 | Nov 14, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-001 | Nov 14, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-001 | Nov 14, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | BANZEL | rufinamide | SUSPENSION;ORAL | 201367-001 | Mar 3, 2011 | ⤷ Try a Trial | ⤷ Try a Trial |
| Eisai Inc | BANZEL | rufinamide | TABLET;ORAL | 021911-002 | Nov 14, 2008 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for rufinamide
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Eisai GmbH | Inovelon | rufinamide | EMEA/H/C/000660 Inovelon is indicated as adjunctive therapy in the treatment of seizures associated with Lennox Gastaut syndrome in patients 4 years of age and older. |
Authorised | no | no | no | 2007-01-16 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |