Isosorbide - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for isosorbide and what is the scope of patent protection?
Isosorbide
is the generic ingredient in nine branded drugs marketed by Alcon, Auxilium Pharms Llc, Wyeth Ayerst, Astrazeneca, Impax Labs Inc, Sun Pharm Inds Inc, Bausch, Ani Pharms, Hikma Intl Pharms, Par Pharm, Par Pharm Inc, Rubicon, Sandoz, Sun Pharm Industries, Superpharm, Watson Labs, Zydus, Biovail, Watson Labs Teva, Schering Plough, Accord Hlthcare, Actavis Elizabeth, Alkermes Gainesville, Aurobindo Pharma, Chartwell Molecular, Dexcel Ltd, Ivax Sub Teva Pharms, Riconpharma Llc, Shandong, Skyepharma Ag, Strides Pharma, Torrent Pharms, Zydus Hlthcare, Zydus Pharms, Promius Pharma, Hikma Pharms, and Omnivium Pharms, and is included in sixty-eight NDAs. Additional information is available in the individual branded drug profile pages.There are thirty-seven drug master file entries for isosorbide.
Summary for isosorbide
| US Patents: | 0 |
| Tradenames: | 9 |
| Applicants: | 37 |
| NDAs: | 68 |
| Drug Master File Entries: | 37 |
| Raw Ingredient (Bulk) Api Vendors: | 86 |
| Clinical Trials: | 74 |
| Patent Applications: | 6,726 |
| Formulation / Manufacturing: | see details |
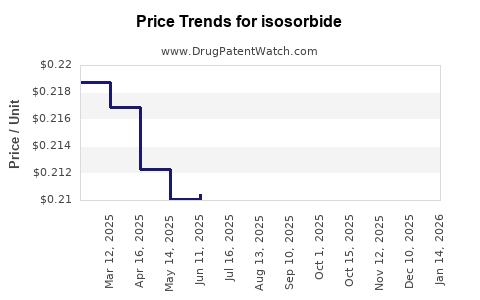
| Drug Prices: | Drug price trends for isosorbide |
| DailyMed Link: | isosorbide at DailyMed |
Recent Clinical Trials for isosorbide
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Zealand University Hospital | Phase 4 |
| Copenhagen University Hospital, Hvidovre | Phase 4 |
| Johannes Grand | Phase 4 |
Medical Subject Heading (MeSH) Categories for isosorbide
US Patents and Regulatory Information for isosorbide
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Astrazeneca | SORBITRATE | isosorbide dinitrate | TABLET;ORAL | 016192-001 | Apr 1, 1996 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Par Pharm | ISOSORBIDE DINITRATE | isosorbide dinitrate | TABLET;ORAL | 087946-001 | Jan 12, 1988 | AB | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Hikma Intl Pharms | ISOSORBIDE DINITRATE | isosorbide dinitrate | TABLET;ORAL | 040591-001 | Jan 10, 2007 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Riconpharma Llc | ISOSORBIDE MONONITRATE | isosorbide mononitrate | TABLET, EXTENDED RELEASE;ORAL | 210918-002 | Nov 5, 2018 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |