Imiquimod - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for imiquimod and what is the scope of freedom to operate?
Imiquimod
is the generic ingredient in three branded drugs marketed by Bausch, Apotex Inc, Cosette, Encube, Fougera Pharms, Glenmark Pharms Inc, Padagis Israel, Strides Pharma, and Taro, and is included in eleven NDAs. There are eleven patents protecting this compound and three Paragraph IV challenges. Additional information is available in the individual branded drug profile pages.Imiquimod has fifty-eight patent family members in thirty-one countries.
There are fourteen drug master file entries for imiquimod. Eight suppliers are listed for this compound.
Summary for imiquimod
| International Patents: | 58 |
| US Patents: | 11 |
| Tradenames: | 3 |
| Applicants: | 9 |
| NDAs: | 11 |
| Drug Master File Entries: | 14 |
| Finished Product Suppliers / Packagers: | 8 |
| Raw Ingredient (Bulk) Api Vendors: | 164 |
| Clinical Trials: | 172 |
| Patent Applications: | 6,703 |
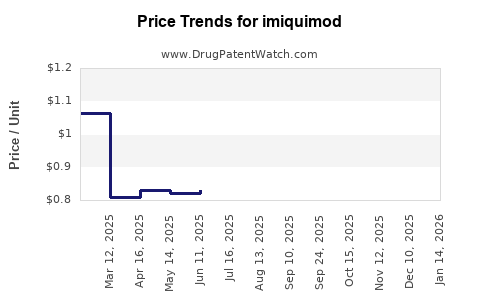
| Drug Prices: | Drug price trends for imiquimod |
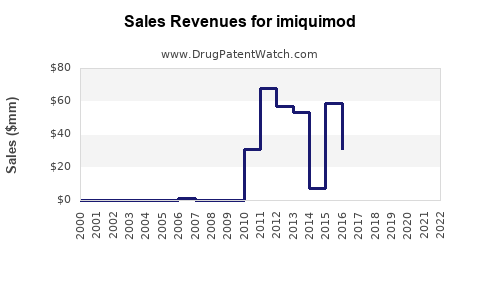
| Drug Sales Revenues: | Drug sales revenues for imiquimod |
| What excipients (inactive ingredients) are in imiquimod? | imiquimod excipients list |
| DailyMed Link: | imiquimod at DailyMed |
Recent Clinical Trials for imiquimod
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Medical University of Vienna | N/A |
| Northwestern University | Early Phase 1 |
| Assiut University | Early Phase 1 |
Pharmacology for imiquimod
| Mechanism of Action | Interferon Inducers |
| Physiological Effect | Increased Cytokine Activity Increased Cytokine Production |
Anatomical Therapeutic Chemical (ATC) Classes for imiquimod
US Patents and Regulatory Information for imiquimod
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bausch | ZYCLARA | imiquimod | CREAM;TOPICAL | 022483-002 | Jul 15, 2011 | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| Bausch | ZYCLARA | imiquimod | CREAM;TOPICAL | 022483-001 | Mar 25, 2010 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Bausch | ZYCLARA | imiquimod | CREAM;TOPICAL | 022483-001 | Mar 25, 2010 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Bausch | ZYCLARA | imiquimod | CREAM;TOPICAL | 022483-001 | Mar 25, 2010 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Bausch | ZYCLARA | imiquimod | CREAM;TOPICAL | 022483-001 | Mar 25, 2010 | AB | RX | Yes | Yes | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | |||
| Cosette | IMIQUIMOD | imiquimod | CREAM;TOPICAL | 200481-001 | Apr 18, 2011 | DISCN | No | No | ⤷ Sign Up | ⤷ Sign Up | ⤷ Sign Up | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for imiquimod
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Bausch | ALDARA | imiquimod | CREAM;TOPICAL | 020723-001 | Feb 27, 1997 | ⤷ Sign Up | ⤷ Sign Up |
| Bausch | ALDARA | imiquimod | CREAM;TOPICAL | 020723-001 | Feb 27, 1997 | ⤷ Sign Up | ⤷ Sign Up |
| Bausch | ALDARA | imiquimod | CREAM;TOPICAL | 020723-001 | Feb 27, 1997 | ⤷ Sign Up | ⤷ Sign Up |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
EU/EMA Drug Approvals for imiquimod
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Viatris Healthcare Limited | Aldara | imiquimod | EMEA/H/C/000179 Imiquimod cream is indicated for the topical treatment of :External genital and perianal warts (condylomata acuminata) in adults.Small superficial basal cell carcinomas (sBCCs) in adults.Clinically typical, nonhyperkeratotic, nonhypertrophic actinic keratoses (AKs) on the face or scalp in immunocompetent adult patients when size or number of lesions limit the efficacy and/or acceptability of cryotherapy and other topical treatment options are contraindicated or less appropriate. |
Authorised | no | no | no | 1998-09-18 | |
| Viatris Healthcare Limited | Zyclara | imiquimod | EMEA/H/C/002387 Zyclara is indicated for the topical treatment of clinically typical, non-hyperkeratotic, non-hypertrophic, visible or palpable actinic keratosis of the full face or balding scalp in immunocompetent adults when other topical treatment options are contraindicated or less appropriate. |
Authorised | no | no | no | 2012-08-23 | |
| Laboratoires 3M Santé | Zartra | imiquimod | EMEA/H/C/000180 Imiquimod cream is indicated for the topical treatment of external genital and perianal warts (condyloma acuminata) in adult patients. |
Withdrawn | no | no | no | 1998-09-18 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for imiquimod
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 2378876 | ⤷ Sign Up | |
| Spain | 2720149 | ⤷ Sign Up | |
| Taiwan | 201040173 | Lower dosage strength imiquimod formulations and short dosing regimens for treating actinic keratosis | ⤷ Sign Up |
| Dominican Republic | P2011000195 | FORMULACIONES DE IMIQUIMOD DE BAJA CONCENTRACION DE DOSIS Y REGIMENES DE DOSIS DE CORTA DURACION PARA TRATAR QUERATOSIS ACTINICA | ⤷ Sign Up |
| Turkey | 201902178 | ⤷ Sign Up | |
| Croatia | P20190306 | ⤷ Sign Up | |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for imiquimod
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0145340 | SPC/GB99/003 | United Kingdom | ⤷ Sign Up | PRODUCT NAME: IMIQUIMOD; REGISTERED: UK EU/1/98/080/001 19980918 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.