Hydrocortisone - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for hydrocortisone and what is the scope of patent protection?
Hydrocortisone
is the generic ingredient in seventy-six branded drugs marketed by Allergan Herbert, Crown Labs, Salix Pharms, Bayer Pharms, Monarch Pharms, Valeant Pharm Intl, Westwood Squibb, Pharm Assoc, C And M Pharma, Actavis Mid Atlantic, Alpharma Us Pharms, Altana, Ambix, Chartwell Molecular, Encube, Everylife, Fougera Pharms Inc, G And W Labs, Ingram Pharm, Ivax Pharms, Naska, Padagis Us, Perrigo New York, Pharmaderm, Pharmafair, Rising, Stiefel, Syosset, Taro, Teva, Topiderm, Usl Pharma, Whiteworth Town Plsn, Valeant Intl, Bausch, Chartwell, Ani Pharms, Teva Pharms, Healthpoint, Eton, Pharmacia And Upjohn, Baker Norton, Legacy Pharma, Solvay, Beta Dermac, Bluline, Heran, Fougera Pharms, Mericon, Dow Pharm, Pfizer Global, Cmp Pharma Inc, Dermik Labs, Torch, X Gen Pharms, Paddock Llc, Mission Pharma, Pfizer, Aurobindo Pharma Ltd, Barr, Elkins Sinn, Ferrante, Hikma Intl Pharms, Impax Labs, Impax Labs Inc, Inwood Labs, Nexgen Pharma Inc, Panray, Parke Davis, Purepac Pharm, Roxane, Sandoz, Strides Pharma, Watson Labs, Merck, Pfipharmecs, Mylan Speciality Lp, Able, Cenci, Imperium, Sebela Ireland Ltd, Bel Mar, Epic Pharma Llc, Fera Pharms, Colgate, Genus, Ferndale Labs, Glenmark Generics, Taro Pharm Inds, Yamanouchi, Precision Dermat, Lupin Ltd, The J Molner, Abbott, Hospira, Abraxis Pharm, Baxter Hlthcare, Intl Medication, Cosette, Encube Ethicals, Glenmark Pharms Ltd, Padagis Israel, Sun Pharm Inds Inc, Amring Pharms, Bausch And Lomb, Casper Pharma Llc, Schering, Actavis Labs Fl Inc, Forest Labs, Lederle, and Bioglan, and is included in two hundred and fifty-eight NDAs. There are four patents protecting this compound. Additional information is available in the individual branded drug profile pages.Hydrocortisone has fifty-six patent family members in twenty-five countries.
There are sixty-seven drug master file entries for hydrocortisone. Thirty-six suppliers are listed for this compound.
Summary for hydrocortisone
| International Patents: | 56 |
| US Patents: | 4 |
| Tradenames: | 76 |
| Applicants: | 111 |
| NDAs: | 258 |
| Drug Master File Entries: | 67 |
| Finished Product Suppliers / Packagers: | 36 |
| Raw Ingredient (Bulk) Api Vendors: | 109 |
| Clinical Trials: | 436 |
| Patent Applications: | 7,189 |
| Formulation / Manufacturing: | see details |
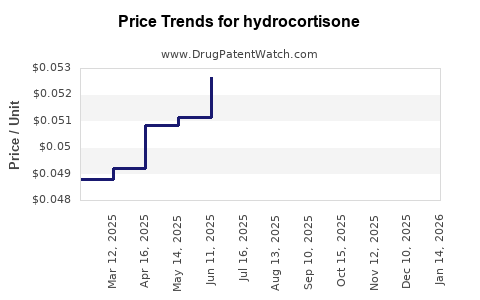
| Drug Prices: | Drug price trends for hydrocortisone |
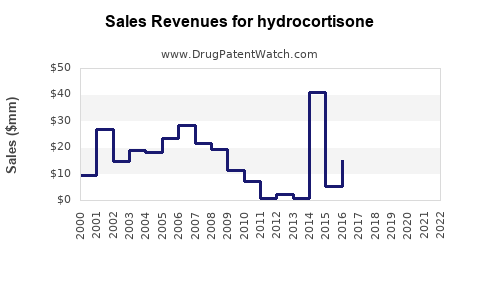
| Drug Sales Revenues: | Drug sales revenues for hydrocortisone |
| What excipients (inactive ingredients) are in hydrocortisone? | hydrocortisone excipients list |
| DailyMed Link: | hydrocortisone at DailyMed |
Recent Clinical Trials for hydrocortisone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| The Gothenburg Society of Medicine | N/A |
| Åke Wibergs Stiftelse | N/A |
| Sahlgrenska University Hospital, Sweden | N/A |
Pharmacology for hydrocortisone
| Drug Class | Corticosteroid |
| Mechanism of Action | Corticosteroid Hormone Receptor Agonists |
Medical Subject Heading (MeSH) Categories for hydrocortisone
US Patents and Regulatory Information for hydrocortisone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Padagis Us | STIE-CORT | hydrocortisone | LOTION;TOPICAL | 089074-001 | Nov 26, 1985 | AT | RX | No | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Purepac Pharm | HYDROCORTISONE | hydrocortisone | TABLET;ORAL | 084247-003 | Aug 31, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Westwood Squibb | FLEXICORT | hydrocortisone | CREAM;TOPICAL | 087136-002 | Apr 8, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pharmaderm | HYDROCORTISONE | hydrocortisone | OINTMENT;TOPICAL | 088842-001 | Feb 9, 1987 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
EU/EMA Drug Approvals for hydrocortisone
| Company | Drugname | Inn | Product Number / Indication | Status | Generic | Biosimilar | Orphan | Marketing Authorisation | Marketing Refusal |
|---|---|---|---|---|---|---|---|---|---|
| Takeda Pharmaceuticals International AG Ireland Branch | Plenadren | hydrocortisone | EMEA/H/C/002185 Treatment of adrenal insufficiency in adults. |
Authorised | no | no | no | 2011-11-03 | |
| Diurnal Europe B.V. | Alkindi | hydrocortisone | EMEA/H/C/004416 Replacement therapy of adrenal insufficiency in infants, children and adolescents (from birth to < 18 years old). |
Authorised | no | no | no | 2018-02-09 | |
| Diurnal Europe B.V. | Efmody | hydrocortisone | EMEA/H/C/005105 Treatment of congenital adrenal hyperplasia (CAH) in adolescents aged 12 years and over and adults. |
Authorised | no | no | no | 2021-05-27 | |
| >Company | >Drugname | >Inn | >Product Number / Indication | >Status | >Generic | >Biosimilar | >Orphan | >Marketing Authorisation | >Marketing Refusal |
International Patents for hydrocortisone
| Country | Patent Number | Title | Estimated Expiration |
|---|---|---|---|
| Denmark | 2978414 | ⤷ Try a Trial | |
| Japan | 2014533679 | 副腎機能障害の治療 | ⤷ Try a Trial |
| South Africa | 201507210 | COMPOSITION COMPRISING HYDROCORTISONE | ⤷ Try a Trial |
| South Korea | 20140108638 | TREATMENT OF ADRENAL INSUFFICIENCY | ⤷ Try a Trial |
| >Country | >Patent Number | >Title | >Estimated Expiration |
Supplementary Protection Certificates for hydrocortisone
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0809498 | 10C0038 | France | ⤷ Try a Trial | PRODUCT NAME: ACYCLOVIR ET HYDROCORTISONE; NAT. REGISTRATION NO/DATE: NL 36 826 20100420; FIRST REGISTRATION: SK - 2108/08467-R 20091026 |
| 0809498 | SPC/GB10/012 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: A COMBINATION OF ACYCLOVIR AND HYDROCORTISONE; REGISTERED: UK PL18191/0001-0001 20091112 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Make Better Decisions: Try a trial or see plans & pricing
Drugs may be covered by multiple patents or regulatory protections. All trademarks and applicant names are the property of their respective owners or licensors. Although great care is taken in the proper and correct provision of this service, thinkBiotech LLC does not accept any responsibility for possible consequences of errors or omissions in the provided data. The data presented herein is for information purposes only. There is no warranty that the data contained herein is error free. thinkBiotech performs no independent verification of facts as provided by public sources nor are attempts made to provide legal or investing advice. Any reliance on data provided herein is done solely at the discretion of the user. Users of this service are advised to seek professional advice and independent confirmation before considering acting on any of the provided information. thinkBiotech LLC reserves the right to amend, extend or withdraw any part or all of the offered service without notice.