Chlorpromazine - Generic Drug Details
✉ Email this page to a colleague
What are the generic sources for chlorpromazine and what is the scope of patent protection?
Chlorpromazine
is the generic ingredient in five branded drugs marketed by Glaxosmithkline, Actavis Mid Atlantic, Genus, Pharm Assoc, Wockhardt, Hikma, Fosun Pharma, Abraxis Pharm, Dr Reddys, Eugia Pharma, Marsam Pharms Llc, Watson Labs, West-ward Pharms Int, Wyeth Ayerst, Alpharma Us Pharms, Abbott, Alembic, Amneal Pharms Co, Appco, Cycle, Glenmark Pharms Ltd, Ivax Sub Teva Pharms, Kv Pharm, Lannett Co Inc, Lederle, Lupin, MSN, Purepac Pharm, Pvt Form, Sandoz, Sun Pharm, Teva Pharms, Upsher Smith Labs, Vangard, West Ward, Zameer Pharms, Zydus, and Parke Davis, and is included in seventy-six NDAs. Additional information is available in the individual branded drug profile pages.There are twenty-four drug master file entries for chlorpromazine.
Summary for chlorpromazine
| US Patents: | 0 |
| Tradenames: | 5 |
| Applicants: | 38 |
| NDAs: | 76 |
| Drug Master File Entries: | 24 |
| Raw Ingredient (Bulk) Api Vendors: | 74 |
| Clinical Trials: | 36 |
| Patent Applications: | 6,969 |
| Formulation / Manufacturing: | see details |
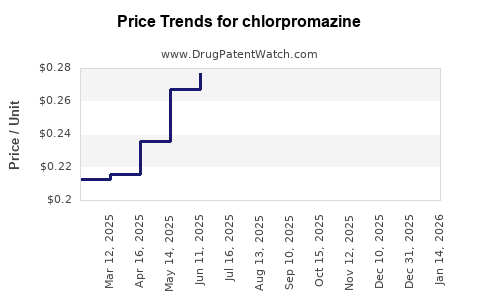
| Drug Prices: | Drug price trends for chlorpromazine |
| What excipients (inactive ingredients) are in chlorpromazine? | chlorpromazine excipients list |
| DailyMed Link: | chlorpromazine at DailyMed |
Recent Clinical Trials for chlorpromazine
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Cancer Prevention Research Institute of Texas | Phase 2/Phase 3 |
| Sadat City University | Phase 1/Phase 2 |
| Varun Monga, MD | Phase 1 |