Betamethasone - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for betamethasone and what is the scope of freedom to operate?
Betamethasone
is the generic ingredient in twenty-six branded drugs marketed by Schering, Merck Sharp Dohme, Am Regent, Hikma, Organon, Parke Davis, Anda Repository, Fougera Pharms, Glenmark Generics, Padagis Israel, Taro, Savage Labs, Actavis Mid Atlantic, Cosette, Perrigo New York, Pharmaderm, Teva, Zydus Pharms, Encube, Alpharma Us Pharms, Fougera Pharms Inc, Padagis Us, Shree Hari Intl, Lupin Ltd, Tasman Pharma, Zydus Lifesciences, Primus Pharms, Glenmark Pharms Ltd, Leo Pharma As, MC2, Chartwell Rx, Glenmark Pharms, Watson Labs, Novast Labs, Xiromed, Mylan, Roaco, Pharmafair, Anima, and Teva Pharms, and is included in one hundred and twenty NDAs. There are seventeen patents protecting this compound. Additional information is available in the individual branded drug profile pages.There are sixty-six drug master file entries for betamethasone.
Summary for betamethasone
| US Patents: | 17 |
| Tradenames: | 26 |
| Applicants: | 40 |
| NDAs: | 120 |
| Drug Master File Entries: | 66 |
| Raw Ingredient (Bulk) Api Vendors: | 79 |
| Clinical Trials: | 195 |
| Patent Applications: | 6,594 |
| Formulation / Manufacturing: | see details |
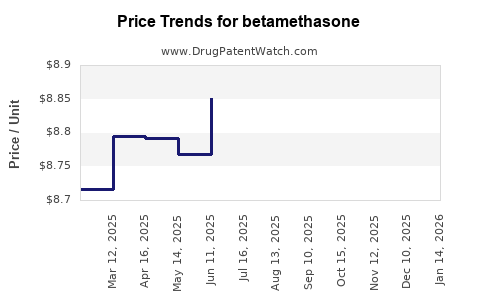
| Drug Prices: | Drug price trends for betamethasone |
| Drug Sales Revenues: | Drug sales revenues for betamethasone |
| DailyMed Link: | betamethasone at DailyMed |
Recent Clinical Trials for betamethasone
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Oticara Australia PTY LTD | Phase 2 |
| Beni-Suef University | Phase 4 |
| Assiut University | Phase 4 |
Medical Subject Heading (MeSH) Categories for betamethasone
US Patents and Regulatory Information for betamethasone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Taro | DERMABET | betamethasone valerate | CREAM;TOPICAL | 072041-001 | Jan 6, 1988 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Anda Repository | BETAMETHASONE DIPROPIONATE | betamethasone dipropionate | CREAM, AUGMENTED;TOPICAL | 076603-001 | Jan 23, 2004 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Pharmaderm | BETAMETHASONE VALERATE | betamethasone valerate | OINTMENT;TOPICAL | 018864-001 | Aug 31, 1983 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Actavis Mid Atlantic | BETAMETHASONE DIPROPIONATE | betamethasone dipropionate | OINTMENT;TOPICAL | 071012-001 | Feb 3, 1987 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Mylan | LUXIQ | betamethasone valerate | AEROSOL, FOAM;TOPICAL | 020934-001 | Feb 28, 1999 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Padagis Israel | BETAMETHASONE VALERATE | betamethasone valerate | AEROSOL, FOAM;TOPICAL | 078337-001 | Nov 26, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for betamethasone
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Schering | CELESTONE | betamethasone | TABLET;ORAL | 012657-003 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Schering | CELESTONE | betamethasone | CREAM;TOPICAL | 014762-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Merck Sharp Dohme | CELESTONE | betamethasone | SYRUP;ORAL | 014215-002 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |