Albuterol - Generic Drug Details
✉ Email this page to a colleague
What are the generic drug sources for albuterol and what is the scope of freedom to operate?
Albuterol
is the generic ingredient in eighteen branded drugs marketed by Armstrong Pharms, Genpharm, Ivax Sub Teva Pharms, Pliva, Schering, Glaxosmithkline, Cipla, Lupin, Padagis Us, Sandoz, Teva Branded Pharm, Kindeva, Mylan Speciality Lp, Actavis Mid Atlantic, Apotex Inc, Bausch, Copley Pharm, Epic Pharma Llc, Landela Pharm, Luoxin Aurovitas, Mylan Speclt, Nephron, Ritedose Corp, Roxane, Sentiss, Sun Pharm, Teva Pharms, Watson Labs, Watson Labs Inc, Wockhardt Eu Operatn, Amneal Pharms, Chartwell Molecular, Chartwell Rx, Cosette, Hikma, Mova, Quagen, Teva, Rising, Muro, Strides Pharma, Aizant, Am Therap, Amneal Pharms Co, Aurobindo Pharma Ltd, Dash Pharms Natco, Eywa, Sun Pharm Industries, Ucb Inc, Virtus Pharm, Warner Chilcott, Zydus Pharms, Astrazeneca, Boehringer Ingelheim, Fosun Pharma, and Watson Labs Teva, and is included in one hundred and six NDAs. There are thirty-five patents protecting this compound. Additional information is available in the individual branded drug profile pages.There are thirty-eight drug master file entries for albuterol. There is one tentative approval for this compound.
Summary for albuterol
| US Patents: | 35 |
| Tradenames: | 18 |
| Applicants: | 56 |
| NDAs: | 106 |
| Drug Master File Entries: | 38 |
| Raw Ingredient (Bulk) Api Vendors: | 133 |
| Clinical Trials: | 282 |
| Patent Applications: | 6,615 |
| Formulation / Manufacturing: | see details |
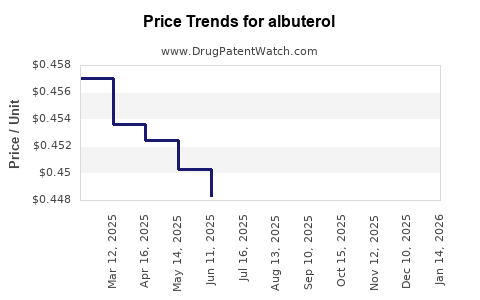
| Drug Prices: | Drug price trends for albuterol |
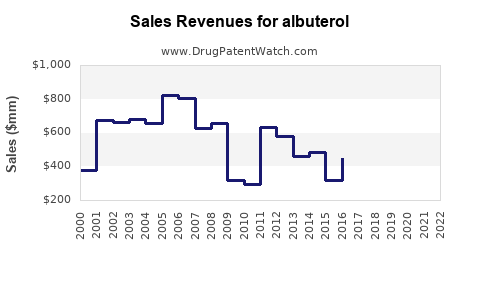
| Drug Sales Revenues: | Drug sales revenues for albuterol |
| What excipients (inactive ingredients) are in albuterol? | albuterol excipients list |
| DailyMed Link: | albuterol at DailyMed |
Recent Clinical Trials for albuterol
Identify potential brand extensions & 505(b)(2) entrants
| Sponsor | Phase |
|---|---|
| Johns Hopkins University | Phase 3 |
| Parexel | Phase 3 |
| Norwegian School of Sport Sciences | N/A |
Generic filers with tentative approvals for ALBUTEROL
| Applicant | Application No. | Strength | Dosage Form |
| ⤷ Try a Trial | ⤷ Try a Trial | EQ 0.083% BASE;0.017% | SOLUTION;INHALATION |
The 'tentative' approval signifies that the product meets all FDA standards for marketing, and, but for the patents / regulatory protections, it would approved.
Medical Subject Heading (MeSH) Categories for albuterol
US Patents and Regulatory Information for albuterol
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Teva Branded Pharm | PROAIR RESPICLICK | albuterol sulfate | POWDER, METERED;INHALATION | 205636-001 | Mar 31, 2015 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Glaxosmithkline | VENTOLIN ROTACAPS | albuterol sulfate | CAPSULE;INHALATION | 019489-001 | May 4, 1988 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Teva Branded Pharm | PROAIR HFA | albuterol sulfate | AEROSOL, METERED;INHALATION | 021457-001 | Oct 29, 2004 | AB2 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Boehringer Ingelheim | COMBIVENT RESPIMAT | albuterol sulfate; ipratropium bromide | SPRAY, METERED;INHALATION | 021747-001 | Oct 7, 2011 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Glaxosmithkline | VENTOLIN | albuterol sulfate | SOLUTION;INHALATION | 019269-002 | Jan 16, 1987 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for albuterol
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Glaxosmithkline | VENTOLIN | albuterol | AEROSOL, METERED;INHALATION | 018473-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Glaxosmithkline | VENTOLIN | albuterol | AEROSOL, METERED;INHALATION | 018473-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Schering | PROVENTIL | albuterol | AEROSOL, METERED;INHALATION | 017559-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| Schering | PROVENTIL | albuterol | AEROSOL, METERED;INHALATION | 017559-001 | Approved Prior to Jan 1, 1982 | ⤷ Try a Trial | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |