Formulation information for generic ingredient: abacavir sulfate; dolutegravir sodium; lamivudine
✉ Email this page to a colleague
ABACAVIR SULFATE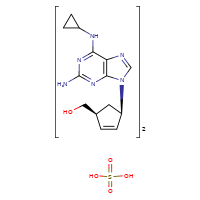
CASRN: 188062-50-2
Manufacturing Methods
…<truncated in preview>
Dolutegravir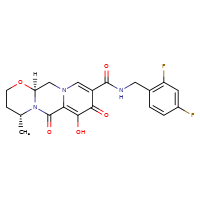
CASRN: 1051375-16-6
Manufacturing Methods
…<truncated in preview>
LAMIVUDINE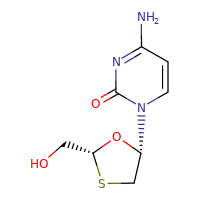
CASRN: 134678-17-4


