Drug Price Trends for ORILISSA
✉ Email this page to a colleague
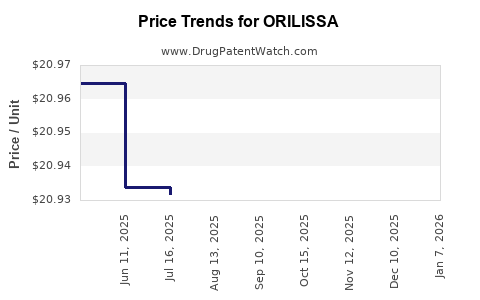
Average Pharmacy Cost for ORILISSA
| Drug Name | NDC | Price/Unit ($) | Unit | Date |
|---|---|---|---|---|
| ORILISSA 200 MG TABLET | 00074-0039-56 | 19.81791 | EACH | 2024-01-02 |
| ORILISSA 150 MG TABLET | 00074-0038-01 | 39.85018 | EACH | 2024-01-02 |
| ORILISSA 150 MG TABLET | 00074-0038-28 | 39.85018 | EACH | 2024-01-02 |
| ORILISSA 200 MG TABLET | 00074-0039-01 | 19.81791 | EACH | 2024-01-02 |
| ORILISSA 150 MG TABLET | 00074-0038-28 | 37.95256 | EACH | 2023-10-18 |
| ORILISSA 200 MG TABLET | 00074-0039-01 | 18.87420 | EACH | 2023-10-18 |
| >Drug Name | >NDC | >Price/Unit ($) | >Unit | >Date |
Best Wholesale Price for ORILISSA
| Drug Name | Vendor | NDC | Count | Price ($) | Price/Unit ($) | Unit | Dates | Price Type |
|---|---|---|---|---|---|---|---|---|
| ORILISSA 150MG TAB | Abbvie US LLC | 00074-0038-28 | 4X7 | 828.45 | 2024-01-01 - 2024-12-31 | FSS | ||
| ORILISSA 200MG TAB | Abbvie US LLC | 00074-0039-56 | 4X14 | 802.80 | 2024-01-01 - 2024-12-31 | Big4 | ||
| ORILISSA 200MG TAB | Abbvie US LLC | 00074-0039-56 | 4X14 | 828.45 | 2024-01-01 - 2024-12-31 | FSS | ||
| ORILISSA 150MG TAB | Abbvie US LLC | 00074-0038-28 | 4X7 | 801.79 | 2024-01-01 - 2024-12-31 | Big4 | ||
| >Drug Name | >Vendor | >NDC | >Count | >Price ($) | >Price/Unit ($) | >Unit | >Dates | >Price Type |


