Noven Company Profile
✉ Email this page to a colleague
What is the competitive landscape for NOVEN, and what generic alternatives to NOVEN drugs are available?
NOVEN has eight approved drugs.
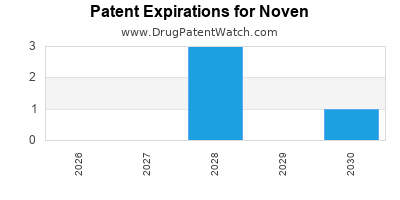
There are eleven US patents protecting NOVEN drugs. There are three tentative approvals on NOVEN drugs.
There are fifty-four patent family members on NOVEN drugs in eighteen countries and fifty-two supplementary protection certificates in fourteen countries.
Summary for Noven
| International Patents: | 54 |
| US Patents: | 11 |
| Tradenames: | 11 |
| Ingredients: | 7 |
| NDAs: | 8 |
| Patent Litigation for Noven: | See patent lawsuits for Noven |
| PTAB Cases with Noven as petitioner: | See PTAB cases with Noven as petitioner |
Drugs and US Patents for Noven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-003 | Apr 6, 2006 | AB | RX | Yes | No | 9,034,370 | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-004 | Mar 22, 2022 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-001 | Apr 6, 2006 | AB | RX | Yes | No | 9,668,981 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-001 | Mar 22, 2022 | RX | Yes | No | 9,034,370 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-003 | Oct 29, 2012 | AB3 | RX | Yes | No | 8,231,906 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| Noven Pharms Inc | XELSTRYM | dextroamphetamine | SYSTEM;TRANSDERMAL | 215401-003 | Mar 22, 2022 | RX | Yes | No | 9,474,722 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Noven
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Noven Pharms Inc | COMBIPATCH | estradiol; norethindrone acetate | FILM, EXTENDED RELEASE;TRANSDERMAL | 020870-002 | Aug 7, 1998 | 5,958,446 | ⤷ Try a Trial |
| Noven | DENTIPATCH | lidocaine | FILM, EXTENDED RELEASE;BUCCAL | 020575-001 | May 21, 1996 | 5,446,070 | ⤷ Try a Trial |
| Noven Pharms Inc | COMBIPATCH | estradiol; norethindrone acetate | FILM, EXTENDED RELEASE;TRANSDERMAL | 020870-001 | Aug 7, 1998 | 5,958,446 | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-005 | Sep 23, 2014 | 6,841,716 | ⤷ Try a Trial |
| Noven | MINIVELLE | estradiol | FILM, EXTENDED RELEASE;TRANSDERMAL | 203752-003 | Oct 29, 2012 | 6,024,976 | ⤷ Try a Trial |
| Noven Pharms Inc | DAYTRANA | methylphenidate | FILM, EXTENDED RELEASE;TRANSDERMAL | 021514-004 | Apr 6, 2006 | 5,958,446 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for NOVEN drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Transdermal System | 0.025 mg/day | ➤ Subscribe | 2015-05-08 |
| ➤ Subscribe | Transdermal System | 0.0375 mg/day, 0.05 mg/day, 0.075 mg/day, 0.1 mg/day | ➤ Subscribe | 2014-08-18 |
| ➤ Subscribe | Transdermal System | 10 mg/9 hrs, 15 mg/9 hrs, 20 mg/9 hrs and 30 mg/9 hrs | ➤ Subscribe | 2011-04-13 |
International Patents for Noven Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 053309 | ⤷ Try a Trial |
| Taiwan | 201427723 | ⤷ Try a Trial |
| Russian Federation | 2011101477 | ⤷ Try a Trial |
| Spain | 2596809 | ⤷ Try a Trial |
| Spain | 2548457 | ⤷ Try a Trial |
| Japan | 2011527697 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Noven Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1635783 | 300653 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: FENTANYL IN ELKE DOOR HET BASISOCTROOI BESCHERMDE VERSCHIJNINGSVORM; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100906 |
| 0770388 | 9/2009 | Austria | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION AUS ESTRADIOLVALERAT UND DIENOGEST; NAT. REGISTRATION NO/DATE: 1-28003 20090203; FIRST REGISTRATION: BE BE 327792 20081103 |
| 0383579 | C960030 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: REMIFENTANYLUM, DESGEWENST IN DE VORM VAN EEN ZUURADDITIE-ZOUT, IN HET BIJZONDER HET HYDROCHLORIDE; NAT. REGISTRATION NO/DATE: RVG 20601 - RVG 20603 19961015; 36335.00.00, 36335.01.00, 36335.02.00 19960517 |
| 1214076 | C01214076/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONE + ETHINYLESTRADIOL; REGISTRATION NUMBER/DATE: SWISSMEDIC 57946 13.06.2008 |
| 1453521 | 132016000025143 | Italy | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL ED ETINILESTRADIOLO(SEASONIQUE); AUTHORISATION NUMBER(S) AND DATE(S): 17/0017/15-S, 20150211;042139016, 20150414 |
| 1453521 | 122015000093 | Germany | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL UND ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: 87675.00.00 20150720; FIRST REGISTRATION: SLOWAKEI 17/0017/15-S 20150129 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.