UPJOHN Company Profile
✉ Email this page to a colleague
What is the competitive landscape for UPJOHN, and when can generic versions of UPJOHN drugs launch?
UPJOHN has thirteen approved drugs.
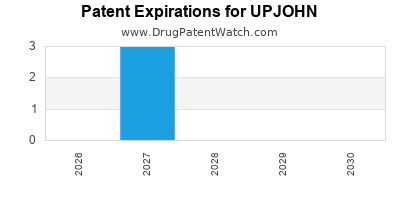
There are three US patents protecting UPJOHN drugs.
There are thirty-three patent family members on UPJOHN drugs in thirty-three countries and thirty-nine supplementary protection certificates in thirteen countries.
Drugs and US Patents for UPJOHN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upjohn | LYRICA | pregabalin | CAPSULE;ORAL | 021446-001 | Dec 30, 2004 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Upjohn | INSPRA | eplerenone | TABLET;ORAL | 021437-003 | Sep 27, 2002 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Upjohn | XANAX XR | alprazolam | TABLET, EXTENDED RELEASE;ORAL | 021434-001 | Jan 17, 2003 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Upjohn | LYRICA CR | pregabalin | TABLET, EXTENDED RELEASE;ORAL | 209501-001 | Oct 11, 2017 | AB | RX | Yes | No | 8,945,620*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| Upjohn | XANAX XR | alprazolam | TABLET, EXTENDED RELEASE;ORAL | 021434-002 | Jan 17, 2003 | AB | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Upjohn | LYRICA CR | pregabalin | TABLET, EXTENDED RELEASE;ORAL | 209501-001 | Oct 11, 2017 | AB | RX | Yes | No | 10,022,447*PED | ⤷ Try a Trial | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for UPJOHN
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Upjohn | CELEBREX | celecoxib | CAPSULE;ORAL | 020998-003 | Aug 29, 2002 | 5,563,165*PED | ⤷ Try a Trial |
| Upjohn | LIPITOR | atorvastatin calcium | TABLET;ORAL | 020702-001 | Dec 17, 1996 | 4,681,893*PED | ⤷ Try a Trial |
| Upjohn | DETROL LA | tolterodine tartrate | CAPSULE, EXTENDED RELEASE;ORAL | 021228-001 | Dec 22, 2000 | 6,630,162*PED | ⤷ Try a Trial |
| Upjohn | CELEBREX | celecoxib | CAPSULE;ORAL | 020998-002 | Dec 31, 1998 | 5,760,068*PED | ⤷ Try a Trial |
| Upjohn | DETROL LA | tolterodine tartrate | CAPSULE, EXTENDED RELEASE;ORAL | 021228-001 | Dec 22, 2000 | 6,770,295*PED | ⤷ Try a Trial |
| Upjohn | EFFEXOR XR | venlafaxine hydrochloride | CAPSULE, EXTENDED RELEASE;ORAL | 020699-002 | Oct 20, 1997 | 4,535,186*PED | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for UPJOHN drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Extended-release Tablets | 37.5 mg, 75 mg and 150 mg | ➤ Subscribe | 2007-05-03 |
| ➤ Subscribe | Capsules | 25 mg, 50 mg, 75 mg, 100 mg, 150 mg, 200 mg, 225 mg and 300 mg | ➤ Subscribe | 2008-12-30 |
| ➤ Subscribe | Extended-release Tablets | 330 mg | ➤ Subscribe | 2018-01-29 |
| ➤ Subscribe | Extended-release Tablets | 330 mg | ➤ Subscribe | 2018-01-29 |
| ➤ Subscribe | Tablets | 25 mg and 50 mg | ➤ Subscribe | 2006-09-27 |
| ➤ Subscribe | Extended-release Capsules | 2 mg and 4 mg | ➤ Subscribe | 2007-07-30 |
| ➤ Subscribe | Capsules | 50 mg | ➤ Subscribe | 2008-03-21 |
| ➤ Subscribe | Oral Solution | 20 mg/mL | ➤ Subscribe | 2010-05-19 |
| ➤ Subscribe | Extended-release Tablets | 82.5 mg and 165 mg | ➤ Subscribe | 2018-02-02 |
| ➤ Subscribe | Extended-release Tablets | 82.5 mg and 165 mg | ➤ Subscribe | 2018-02-02 |
| ➤ Subscribe | Tablets | 20 mg and 40 mg | ➤ Subscribe | 2010-03-29 |
International Patents for UPJOHN Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Costa Rica | 9950 | ⤷ Try a Trial |
| Slovenia | 1945186 | ⤷ Try a Trial |
| Netherlands | 2000281 | ⤷ Try a Trial |
| Israel | 190827 | ⤷ Try a Trial |
| Hong Kong | 1126394 | ⤷ Try a Trial |
| Japan | 2009514847 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for UPJOHN Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0364417 | SPC/GB97/014 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LATANOPROST (I.E. 13,14-DIHYDRO-17-PHENYL-18,19,20-TRINOR-PGF-ALPHA-ISOPROPYLESTER); NAT. REGISTRATION NO/DATE: 00032/0220 19961216; FIRST REGISTRATION: SE 12716 19960718; SPC EXTENSION AUTHORISATION: PL00057/1057-008 20101216 |
| 3461484 | 21C1024 | France | ⤷ Try a Trial | PRODUCT NAME: ASSOCIATION DE NETARSUDIL OU L'UN DE SES SELS ET DE LATANOPROST; REGISTRATION NO/DATE: EU/1/20/1502 20210108 |
| 0720599 | 122014000088 | Germany | ⤷ Try a Trial | PRODUCT NAME: EZETIMIB ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON IN KOMBINATION MIT ATORVASTATIN ODER PHARMAZEUTISCH ANNEHMBARE SALZE DAVON, INSBESONDERE ATORVASTATIN ALS ATORVASTATINCALCIUMTRIHYDRAT; NAT. REGISTRATION NO/DATE: 90223.00.00 20141113; FIRST REGISTRATION: FRANKREICH CIS: 6 928 917 6 20140912 |
| 0592438 | SPC/GB01/023 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: ELETRIPTAN AND PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, IN PARTICULAR THE HYDROBROMIDE SALT; REGISTERED: CH 55218 01 20001214; CH 52218 02 20001214; CH 52218 03 20001214; UK PL 00057/0452 20010212; UK PL 00057/0453 20010212; UK PL 00057/0454 20010212 |
| 0720599 | 300689 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, EN ATORVASTATINE, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT, IN HET BIJZONDER ATORVASTATINE CALCIUM TRIHYDRATE; NATIONAL REGISTRATION NO/DATE: RVG114373-114376 20141027; FIRST REGISTRATION: FR 2014091200122 20140912 |
| 0720599 | CR 2014 00050 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE AND ATORVASTATIN OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING ATORVASTATIN AS ATORVASTATIN CALCIUM TRIHYDRATE; REG. NO/DATE: DE/H/3895-3898/001-004/DC 20140910 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.