UCB INC Company Profile
✉ Email this page to a colleague
What is the competitive landscape for UCB INC, and when can generic versions of UCB INC drugs launch?
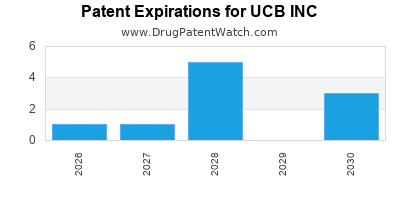
UCB INC has forty-two approved drugs.
There are thirty-four US patents protecting UCB INC drugs.
There are four hundred and eighty-four patent family members on UCB INC drugs in fifty-one countries and thirty-nine supplementary protection certificates in fourteen countries.
Summary for UCB INC
| International Patents: | 484 |
| US Patents: | 34 |
| Tradenames: | 29 |
| Ingredients: | 24 |
| NDAs: | 42 |
| Drug Master File Entries: | 1 |
| Patent Litigation for UCB INC: | See patent lawsuits for UCB INC |
Drugs and US Patents for UCB INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-001 | May 12, 2016 | RX | Yes | No | 6,911,461 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Ucb Inc | FLUXID | famotidine | TABLET, ORALLY DISINTEGRATING;ORAL | 021712-001 | Sep 24, 2004 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-002 | May 12, 2016 | RX | Yes | No | 10,729,653 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Ucb Inc | BRIVIACT | brivaracetam | TABLET;ORAL | 205836-003 | May 12, 2016 | RX | Yes | No | 6,911,461 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for UCB INC
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Ucb Inc | KEPPRA | levetiracetam | TABLET;ORAL | 021035-003 | Nov 30, 1999 | 4,837,223 | ⤷ Try a Trial |
| Ucb Inc | UNIVASC | moexipril hydrochloride | TABLET;ORAL | 020312-001 | Apr 19, 1995 | 4,743,450 | ⤷ Try a Trial |
| Ucb Inc | VIMPAT | lacosamide | SOLUTION;INTRAVENOUS | 022254-001 | Oct 28, 2008 | 5,654,301 | ⤷ Try a Trial |
| Ucb Inc | NEUPRO | rotigotine | FILM, EXTENDED RELEASE;TRANSDERMAL | 021829-004 | Apr 2, 2012 | 6,884,434 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for UCB INC drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 1000 mg | ➤ Subscribe | 2007-01-24 |
| ➤ Subscribe | Tablets | 50 mg, 100 mg, 150 mg, and 200 mg | ➤ Subscribe | 2012-10-29 |
| ➤ Subscribe | Extended-release Tablets | 1000 mg | ➤ Subscribe | 2011-01-07 |
| ➤ Subscribe | Tablets | 7.5mg/12.5mg 15 mg/25 mg and 15 mg/12.5 mg | ➤ Subscribe | 2004-01-15 |
| ➤ Subscribe | Extended-release Transdermal Film | 1 mg/24 hr, 2 mg/24 hr, 3 mg/24 hr,4 mg/24 hr,6 mg/24 hr, and 8 mg/24 hr | ➤ Subscribe | 2013-11-26 |
| ➤ Subscribe | Oral Solution | 10 mg/mL | ➤ Subscribe | 2012-10-29 |
| ➤ Subscribe | Injection | 10 mg/mL, 20 mL | ➤ Subscribe | 2016-06-30 |
| ➤ Subscribe | Orally Disintegrating Tablets | 0.25 mg, 0.5 mg, 1 mg and 2 mg | ➤ Subscribe | 2005-12-27 |
International Patents for UCB INC Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Mexico | 2021000482 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2011076879 | ⤷ Try a Trial |
| China | 1671365 | ⤷ Try a Trial |
| Spain | 2679800 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for UCB INC Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1452524 | 10/2016 | Austria | ⤷ Try a Trial | PRODUCT NAME: BRIVARACETAM; REGISTRATION NO/DATE: EU/1/15/1073 (MITTEILUNG) 20160118 |
| 1429780 | 13C0012 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE CIPROFLOXACINE ET DE DEXAMETHASONE, EN PARTICULIER DE CHLORHYDRATE DE CIPROFLOXACINE ET DE DEXAMETHASONE; NAT. REGISTRATION NO/DATE: NL 41308 20121214; FIRST REGISTRATION: 48976 20120808 |
| 1033978 | 06C0025 | France | ⤷ Try a Trial | PRODUCT NAME: ROTIGOTINE; REGISTRATION NO/DATE: EU/1/05/331/001 20060215 |
| 1452524 | 132016000045699 | Italy | ⤷ Try a Trial | PRODUCT NAME: BRIVARACETAM(BRIVIACT); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/15/1073, 20160118 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.