TOLMAR Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TOLMAR, and when can generic versions of TOLMAR drugs launch?
TOLMAR has nine approved drugs.
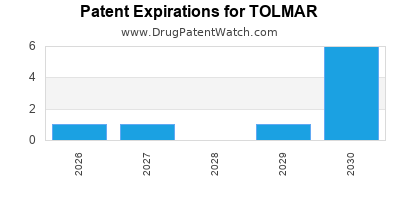
There are eleven US patents protecting TOLMAR drugs. There is one tentative approval on TOLMAR drugs.
There are thirty-seven patent family members on TOLMAR drugs in eighteen countries and two supplementary protection certificates in two countries.
Summary for TOLMAR
| International Patents: | 37 |
| US Patents: | 11 |
| Tradenames: | 6 |
| Ingredients: | 5 |
| NDAs: | 9 |
| Patent Litigation for TOLMAR: | See patent lawsuits for TOLMAR |
Drugs and US Patents for TOLMAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tolmar | JATENZO | testosterone undecanoate | CAPSULE;ORAL | 206089-002 | Mar 27, 2019 | RX | Yes | No | 8,241,664 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Tolmar | RANITIDINE HYDROCHLORIDE | ranitidine hydrochloride | SYRUP;ORAL | 090054-001 | Nov 15, 2010 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Tolmar | JATENZO | testosterone undecanoate | CAPSULE;ORAL | 206089-003 | Mar 27, 2019 | RX | Yes | Yes | 10,617,696 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Tolmar | JATENZO | testosterone undecanoate | CAPSULE;ORAL | 206089-003 | Mar 27, 2019 | RX | Yes | Yes | 11,564,933 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021343-001 | Jan 23, 2002 | RX | Yes | Yes | 11,771,841 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Tolmar | JATENZO | testosterone undecanoate | CAPSULE;ORAL | 206089-003 | Mar 27, 2019 | RX | Yes | Yes | 10,543,219 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tolmar | JATENZO | testosterone undecanoate | CAPSULE;ORAL | 206089-003 | Mar 27, 2019 | RX | Yes | Yes | 11,179,403 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TOLMAR
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021731-001 | Dec 14, 2004 | 6,773,714 | ⤷ Try a Trial |
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021343-001 | Jan 23, 2002 | 5,324,519 | ⤷ Try a Trial |
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021731-001 | Dec 14, 2004 | 5,739,176 | ⤷ Try a Trial |
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021343-001 | Jan 23, 2002 | 5,599,552 | ⤷ Try a Trial |
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021379-001 | Jul 24, 2002 | 5,739,176 | ⤷ Try a Trial |
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021731-001 | Dec 14, 2004 | RE37950 | ⤷ Try a Trial |
| Tolmar | ELIGARD KIT | leuprolide acetate | POWDER;SUBCUTANEOUS | 021343-001 | Jan 23, 2002 | 5,733,950 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TOLMAR Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| European Patent Office | 1871384 | ⤷ Try a Trial |
| Israel | 303673 | ⤷ Try a Trial |
| South Korea | 20230125011 | ⤷ Try a Trial |
| Portugal | 2985026 | ⤷ Try a Trial |
| China | 116847900 | ⤷ Try a Trial |
| Japan | 6251713 | ⤷ Try a Trial |
| Colombia | 2023009588 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for TOLMAR Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0162036 | 2000C/032 | Belgium | ⤷ Try a Trial | PRODUCT NAME: LEVETIRACETAM; NAT. REGISTRATION NO/DATE: EU/1/00/146/001 20000929; FIRST REGISTRATION: CH 55297 20000329 |
| 0162036 | C300028 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVETIRACETAM; REGISTRATION NO/DATE: EU/1/00/146/001 - EU/1/00/146/026 20000929 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.