TERSERA Company Profile
✉ Email this page to a colleague
What is the competitive landscape for TERSERA, and when can generic versions of TERSERA drugs launch?
TERSERA has seven approved drugs.
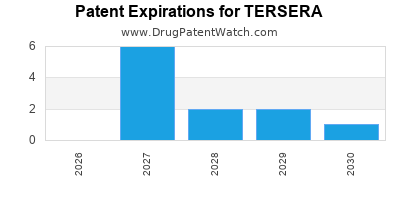
There are seventeen US patents protecting TERSERA drugs.
There are two hundred and twenty-one patent family members on TERSERA drugs in thirty-eight countries and forty-one supplementary protection certificates in eighteen countries.
Drugs and US Patents for TERSERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tersera | VARUBI | rolapitant hydrochloride | TABLET;ORAL | 206500-001 | Sep 1, 2015 | RX | Yes | Yes | 7,981,905 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-004 | Dec 28, 2004 | RX | Yes | Yes | 8,765,680 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tersera | XERMELO | telotristat etiprate | TABLET;ORAL | 208794-001 | Feb 28, 2017 | RX | Yes | Yes | 7,968,559 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-003 | Dec 28, 2004 | DISCN | No | No | 9,707,270 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-002 | Dec 28, 2004 | RX | Yes | Yes | 8,765,680 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tersera | XERMELO | telotristat etiprate | TABLET;ORAL | 208794-001 | Feb 28, 2017 | RX | Yes | Yes | 8,653,094 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Tersera | VARUBI | rolapitant hydrochloride | TABLET;ORAL | 206500-001 | Sep 1, 2015 | RX | Yes | Yes | 7,049,320 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for TERSERA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-001 | Dec 28, 2004 | 5,795,864 | ⤷ Try a Trial |
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-002 | Dec 28, 2004 | 5,364,842 | ⤷ Try a Trial |
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-002 | Dec 28, 2004 | 5,795,864 | ⤷ Try a Trial |
| Tersera | PRIALT | ziconotide acetate | INJECTABLE;INTRATHECAL | 021060-004 | Dec 28, 2004 | 5,859,186 | ⤷ Try a Trial |
| Tersera | VARUBI | rolapitant hydrochloride | EMULSION;INTRAVENOUS | 208399-001 | Oct 25, 2017 | 8,796,299 | ⤷ Try a Trial |
| Tersera | ZOLADEX | goserelin acetate | IMPLANT;IMPLANTATION | 020578-001 | Jan 11, 1996 | 4,100,274 | ⤷ Try a Trial |
| Tersera | ZOLADEX | goserelin acetate | IMPLANT;IMPLANTATION | 019726-001 | Dec 29, 1989 | 7,220,247 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for TERSERA Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Peru | 20142329 | ⤷ Try a Trial |
| Japan | 2010031053 | ⤷ Try a Trial |
| Russian Federation | 2642234 | ⤷ Try a Trial |
| Japan | 2010512416 | ⤷ Try a Trial |
| Colombia | 6220855 | ⤷ Try a Trial |
| Ukraine | 99270 | ⤷ Try a Trial |
| Argentina | 060303 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for TERSERA Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1463716 | 17C1037 | France | ⤷ Try a Trial | PRODUCT NAME: ROLAPITANT, OPTIONNELLEMENT SOUS LA FORME D'UN SEL PHARMACEUTIQUEMENT ADMISSIBLE, INCLUANT LE MONOHYDRATE DE CHLORHYDRATE DE ROLAPITANT.; REGISTRATION NO/DATE: EU/1/17/1180 20170424 |
| 2004646 | 2017C/040 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ROLAPITANT - VARUBY (SOUS FORME DE CHLORHYDRATE MONOHYDRATE); AUTHORISATION NUMBER AND DATE: EU/1/17/1180 20170424 |
| 2091940 | C 2018 007 | Romania | ⤷ Try a Trial | PRODUCT NAME: TELOTRISTAT SAU UN ESTER ACCEPTABIL FARMACEUTIC, SAU O SARE A ACESTUIA, IN PARTICULAR ETIL TELOTRISTAT, MAI PARTICULAR SARE HIPURAT DE ETIL TELOTRISTAT; NATIONAL AUTHORISATION NUMBER: EU/1/17/1224; DATE OF NATIONAL AUTHORISATION: 20170918; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1224; DATE OF FIRST AUTHORISATION IN EEA: 20170918 |
| 1463716 | C20170033 00237 | Estonia | ⤷ Try a Trial | PRODUCT NAME: ROLAPITANT;REG NO/DATE: EU/1/17/1180 24.04.2017 |
| 1463716 | C201730043 | Spain | ⤷ Try a Trial | PRODUCT NAME: ROLAPITANT, OPCIONALMENTE EN FORMA DE UNA SAL FARMACEUTICAMENTE ACEPTABLE, INCLUYENDO ROLAPITANT CLORHIDRATO MONOHIDRATO; NATIONAL AUTHORISATION NUMBER: EU/1/17/1180; DATE OF AUTHORISATION: 20170420; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/17/1180; DATE OF FIRST AUTHORISATION IN EEA: 20170420 |
| 2091940 | 300929 | Netherlands | ⤷ Try a Trial | DETAILS ASSIGNMENT: CHANGE OF OWNER(S), ASSIGNMENT |
| 2091940 | SPC/GB18/009 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: TELOTRISTAT OR A PHARMACEUTICALLY ACCEPTABLE ESTER, OR A SALT THEREOF, IN PARTICULAR TELOTRISTAT ETHYL, MORE PARTICULARLY HIPPURATE SALT OF TELOTRISTAT ETHYL; REGISTERED: UK EU/1/17/1224/001-002 20170920; UK PLGB 28247/0008 20170920 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.