Seagen Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SEAGEN, and what generic alternatives to SEAGEN drugs are available?
SEAGEN has one approved drug.
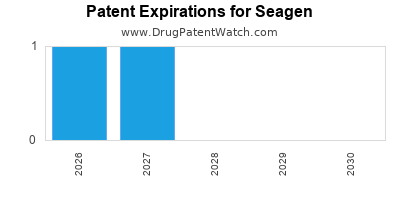
There are seven US patents protecting SEAGEN drugs.
There are one hundred and ninety-one patent family members on SEAGEN drugs in forty-five countries and seventeen supplementary protection certificates in fifteen countries.
Summary for Seagen
| International Patents: | 191 |
| US Patents: | 7 |
| Tradenames: | 1 |
| Ingredients: | 1 |
| NDAs: | 1 |
| Patent Litigation for Seagen: | See patent lawsuits for Seagen |
Drugs and US Patents for Seagen
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | 11,504,370 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-002 | Apr 17, 2020 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-002 | Apr 17, 2020 | RX | Yes | Yes | 7,452,895 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | 8,648,087 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | ||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Seagen | TUKYSA | tucatinib | TABLET;ORAL | 213411-001 | Apr 17, 2020 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
International Patents for Seagen Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Israel | 248993 | ⤷ Try a Trial |
| Australia | 2010202330 | ⤷ Try a Trial |
| European Patent Office | 3615067 | ⤷ Try a Trial |
| European Patent Office | 3842044 | ⤷ Try a Trial |
| Ukraine | 111383 | ⤷ Try a Trial |
| Norway | 336275 | ⤷ Try a Trial |
| Australia | 2012322039 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Seagen Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1971601 | C20210018 00331 | Estonia | ⤷ Try a Trial | PRODUCT NAME: TUKATINIIB;REG NO/DATE: EU/1/20/1526 12.02.2021 |
| 1971601 | C01971601/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: TUCATINIB; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 67798 07.05.2020 |
| 1971601 | C 2021 022 | Romania | ⤷ Try a Trial | PRODUCT NAME: TUCATINIB OPTIONAL SUB FORMA DE SARE ACCEPTABILA FARMACEUTIC SAU SOLVAT; NATIONAL AUTHORISATION NUMBER: EU/1/20/1526; DATE OF NATIONAL AUTHORISATION: 20210211; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): EU/1/20/1526; DATE OF FIRST AUTHORISATION IN EEA: 20210211 |
| 1971601 | 2021C/531 | Belgium | ⤷ Try a Trial | PRODUCT NAME: TUCATINIB, OPTIONEEL IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT OF SOLVAAT; AUTHORISATION NUMBER AND DATE: EU/1/20/1526 20210212 |
| 1971601 | 132021000000128 | Italy | ⤷ Try a Trial | PRODUCT NAME: TUCATINIB OPZIONALMENTE NELLA FORMA DI UN SALE O SOLVATO FARMACEUTICAMENTE ACCETTABILE(TUKYSA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/20/1526, 20210212 |
| 1971601 | CR 2021 00025 | Denmark | ⤷ Try a Trial | PRODUCT NAME: TUCATINIB, EVENTUELT I FORM AF ET FARMACEUTISK ACCEPTABELT SALT ELLER SOLVAT DERAF; REG. NO/DATE: EU/1/20/1526 20210212 |
| 1971601 | 122021000042 | Germany | ⤷ Try a Trial | PRODUCT NAME: TUCATINIB UND SEINE PHYSIOLOGISCH VERTRAEGLICHEN SALZE UND SOLVATE; REGISTRATION NO/DATE: EU/1/20/1526 20210211 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.