SUN PHARM Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SUN PHARM, and what generic alternatives to SUN PHARM drugs are available?
SUN PHARM has five hundred and forty-eight approved drugs.
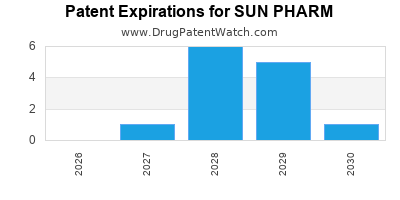
There are thirty-three US patents protecting SUN PHARM drugs. There are eighteen tentative approvals on SUN PHARM drugs.
There are three hundred and seventy-nine patent family members on SUN PHARM drugs in fifty-eight countries and six hundred and nineteen supplementary protection certificates in nineteen countries.
Summary for SUN PHARM
| International Patents: | 379 |
| US Patents: | 33 |
| Tradenames: | 354 |
| Ingredients: | 328 |
| NDAs: | 548 |
| Patent Litigation for SUN PHARM: | See patent lawsuits for SUN PHARM |
| PTAB Cases with SUN PHARM as petitioner: | See PTAB cases with SUN PHARM as petitioner |
Drugs and US Patents for SUN PHARM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun Pharm Inds Ltd | QUINAPRIL HYDROCHLORIDE | quinapril hydrochloride | TABLET;ORAL | 090800-004 | Jun 18, 2009 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sun Pharm Inds Ltd | CIPROFLOXACIN HYDROCHLORIDE | ciprofloxacin hydrochloride | TABLET;ORAL | 075747-003 | Jun 9, 2004 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sun Pharm Inds Inc | LISDEXAMFETAMINE DIMESYLATE | lisdexamfetamine dimesylate | TABLET, CHEWABLE;ORAL | 214134-006 | Aug 25, 2023 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm | ENTACAPONE | entacapone | TABLET;ORAL | 090690-001 | Jul 16, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm | QUETIAPINE FUMARATE | quetiapine fumarate | TABLET;ORAL | 201190-002 | Mar 27, 2012 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Inds | CHILDREN'S FEXOFENADINE HYDROCHLORIDE HIVES | fexofenadine hydrochloride | TABLET;ORAL | 091567-001 | Feb 6, 2012 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SUN PHARM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-001 | May 25, 2012 | 8,952,064 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-001 | May 25, 2012 | 9,078,925 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-003 | May 25, 2012 | 8,952,064 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-001 | May 25, 2012 | 9,089,534 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-003 | May 25, 2012 | 9,078,925 | ⤷ Try a Trial |
| Sun Pharm Industries | BACTRIM | sulfamethoxazole; trimethoprim | TABLET;ORAL | 017377-001 | Approved Prior to Jan 1, 1982 | RE28636 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SUN PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Capsules | 30 mg and 40 mg | ➤ Subscribe | 2012-12-31 |
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-06-19 |
| ➤ Subscribe | Capsules | 25 mg | ➤ Subscribe | 2016-05-16 |
| ➤ Subscribe | Tablets | 125 mg | ➤ Subscribe | 2018-07-23 |
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-01-07 |
| ➤ Subscribe | Capsules | 35 mg | ➤ Subscribe | 2015-11-25 |
International Patents for SUN PHARM Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Hong Kong | 1156628 | ⤷ Try a Trial |
| Cyprus | 1114471 | ⤷ Try a Trial |
| Chile | 2011000551 | ⤷ Try a Trial |
| Spain | 2462946 | ⤷ Try a Trial |
| Honduras | 2008001652 | ⤷ Try a Trial |
| European Patent Office | 3288518 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SUN PHARM Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2822954 | 18C1035 | France | ⤷ Try a Trial | PRODUCT NAME: BICTEGRAVIR OU UN SEL PHARMACEUTIQUEMENT ACCEPTABLE,EN PARTICULIER BICTEGRAVIR DE SODIUM; REGISTRATION NO/DATE: EU/1/18/1289 20180625 |
| 0888289 | SPC/GB09/007 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LACOSAMIDE AND ITS PHARMACEUTICALLY ACCEPTABLE FORMS; REGISTERED: UK EU/1/08/470/001 20080829; UK EU/1/08/470/002 20080829; UK EU/1/08/470/003 20080829; UK EU/1/08/470/004 20080829; UK EU/1/08/470/005 20080829; UK EU/1/08/470/006 20080829; UK EU/1/08/470/014 20080829; UK EU/1/08/470/015 20080829; UK EU/1/08/470/016 20080829; UK EU/1/08/470/007 20080829; UK EU/1/08/470/008 20080829; UK EU/1/08/470/009 20080829; UK EU/1/08/470/010 20080829; UK EU/1/08/470/012 20080829; UK EU/1/08/470/013 20080829 |
| 1429780 | 13C0012 | France | ⤷ Try a Trial | PRODUCT NAME: COMBINAISON DE CIPROFLOXACINE ET DE DEXAMETHASONE, EN PARTICULIER DE CHLORHYDRATE DE CIPROFLOXACINE ET DE DEXAMETHASONE; NAT. REGISTRATION NO/DATE: NL 41308 20121214; FIRST REGISTRATION: 48976 20120808 |
| 2021328 | 255 5026-2015 | Slovakia | ⤷ Try a Trial | FORMER OWNER: NOVARTIS AG, CH |
| 0454511 | 99C0009 | Belgium | ⤷ Try a Trial | PRODUCT NAME: IRBESARTAN / HYDROCHLOROTHIAZIDE; REGISTRATION NO/DATE: EU/1/98/086/001 19981015 |
| 0145340 | 99C0005 | Belgium | ⤷ Try a Trial | PRODUCT NAME: FOSPHENYTOIN DISODIUM; NAT. REGISTRATION NO/DATE: NL 23 613 19980806; FIRST REGISTRATION: GB - PL 000 19/0157 19980204 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.