SUN PHARM Company Profile
✉ Email this page to a colleague
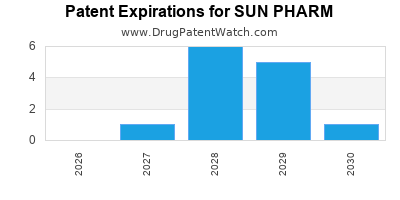
What is the competitive landscape for SUN PHARM, and when can generic versions of SUN PHARM drugs launch?
SUN PHARM has five hundred and forty-eight approved drugs.
There are thirty-three US patents protecting SUN PHARM drugs. There are eighteen tentative approvals on SUN PHARM drugs.
There are three hundred and seventy-nine patent family members on SUN PHARM drugs in fifty-eight countries and six hundred and nineteen supplementary protection certificates in nineteen countries.
Summary for SUN PHARM
| International Patents: | 379 |
| US Patents: | 33 |
| Tradenames: | 354 |
| Ingredients: | 328 |
| NDAs: | 548 |
| Patent Litigation for SUN PHARM: | See patent lawsuits for SUN PHARM |
| PTAB Cases with SUN PHARM as petitioner: | See PTAB cases with SUN PHARM as petitioner |
Drugs and US Patents for SUN PHARM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sun Pharm | LEVETIRACETAM | levetiracetam | TABLET, EXTENDED RELEASE;ORAL | 203059-002 | Sep 9, 2013 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sun Pharm Inds Ltd | MINOCYCLINE HYDROCHLORIDE | minocycline hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 091118-004 | Sep 25, 2014 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sun Pharm Inds Inc | CLONAZEPAM | clonazepam | TABLET, ORALLY DISINTEGRATING;ORAL | 078654-004 | Aug 27, 2014 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Industries | CARVEDILOL PHOSPHATE | carvedilol phosphate | CAPSULE, EXTENDED RELEASE;ORAL | 090132-001 | Oct 25, 2017 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Industries | DOXYCYCLINE HYCLATE | doxycycline hyclate | CAPSULE;ORAL | 062676-002 | Jul 10, 1986 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm | DEFERASIROX | deferasirox | TABLET, FOR SUSPENSION;ORAL | 209782-002 | Nov 20, 2019 | AB | RX | No | No | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sun Pharm Inds Inc | HYDROCHLOROTHIAZIDE | hydrochlorothiazide | CAPSULE;ORAL | 090651-001 | Apr 7, 2014 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SUN PHARM
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sun Pharm Industries | BACTRIM DS | sulfamethoxazole; trimethoprim | TABLET;ORAL | 017377-002 | Approved Prior to Jan 1, 1982 | RE28636 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-006 | Aug 15, 2014 | 9,078,925 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-006 | Aug 15, 2014 | 8,952,064 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-002 | May 25, 2012 | 9,078,925 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-002 | May 25, 2012 | 8,367,102 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-001 | May 25, 2012 | 8,952,064 | ⤷ Try a Trial |
| Sun Pharm Inds Inc | ABSORICA | isotretinoin | CAPSULE;ORAL | 021951-006 | Aug 15, 2014 | 9,089,534 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SUN PHARM drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 20 mg | ➤ Subscribe | 2015-06-03 |
| ➤ Subscribe | Capsules | 30 mg and 40 mg | ➤ Subscribe | 2012-12-31 |
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-06-19 |
| ➤ Subscribe | Capsules | 25 mg | ➤ Subscribe | 2016-05-16 |
| ➤ Subscribe | Tablets | 125 mg | ➤ Subscribe | 2018-07-23 |
| ➤ Subscribe | Capsules | 20 mg | ➤ Subscribe | 2013-01-07 |
| ➤ Subscribe | Capsules | 35 mg | ➤ Subscribe | 2015-11-25 |
International Patents for SUN PHARM Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| World Intellectual Property Organization (WIPO) | 2016051288 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2010102192 | ⤷ Try a Trial |
| Canada | 2736751 | ⤷ Try a Trial |
| South Korea | 101832041 | ⤷ Try a Trial |
| South Korea | 101495192 | ⤷ Try a Trial |
| Japan | 2023103410 | ⤷ Try a Trial |
| Canada | 2907415 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SUN PHARM Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0720599 | CR 2014 00050 | Denmark | ⤷ Try a Trial | PRODUCT NAME: EZETIMIBE AND ATORVASTATIN OR PHARMACEUTICALLY ACCEPTABLE SALTS THEREOF, INCLUDING ATORVASTATIN AS ATORVASTATIN CALCIUM TRIHYDRATE; REG. NO/DATE: DE/H/3895-3898/001-004/DC 20140910 |
| 2316456 | 132017000142109 | Italy | ⤷ Try a Trial | PRODUCT NAME: NALTREXONE/BUPROPIONE(MYSIMBA); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/14/988, 20150330 |
| 0933372 | PA2008006,C0933372 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: FOSAMPRENAVIR CALCIUM; REGISTRATION NO/DATE: EU/1/04/282/001-002 20040712 |
| 1539166 | 2013C/064 | Belgium | ⤷ Try a Trial | PRODUCT NAME: DEXTROMETHORPHANE OU UN SEL, PRCURSEUR DE DERIVE PHARMACEUTIQUEMENT ACCEPTABLE, PAR EXEMPLE LE BROMHYDRURE DE DEXTROMETORPHANE ET EN PARTICULIER LE BROMHYDRURE DE DEXTROMETROPHANE MONHYDRATE ET QUINIDINE OU UN SEL,....; AUTHORISATION NUMBER AND DATE: EU/1/13/833 20130626 |
| 0428296 | SPC/GB98/013 | United Kingdom | ⤷ Try a Trial | PRODUCT NAME: LOPERAMIDE HYDROCHLORIDE AND SIMETHICONE; REGISTERED: UK 00242/0314 19970923 |
| 1261586 | C300524 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: COMBINATIE VAN SAXAGLIPTINE EN METFORMINE, DESGEWENST IN DE VORM VAN FARMACEUTISCH AANVAARDBARE AFGELEIDEN DAARVAN; NAT. REGISTRATION NO/DATE: EU/1/11/731/001-012 20111124; FIRST REGISTRATION: |
| 2021328 | 658 | Finland | ⤷ Try a Trial | |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.