SANOFI AVENTIS US Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANOFI AVENTIS US, and what generic alternatives to SANOFI AVENTIS US drugs are available?
SANOFI AVENTIS US has one hundred and twenty-two approved drugs.
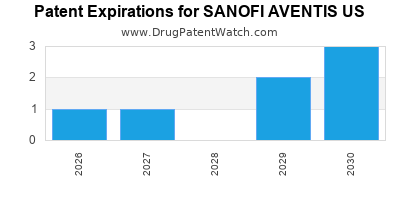
There are ten US patents protecting SANOFI AVENTIS US drugs.
There are two hundred and seventeen patent family members on SANOFI AVENTIS US drugs in fifty-two countries and one hundred and fifteen supplementary protection certificates in nineteen countries.
Summary for SANOFI AVENTIS US
| International Patents: | 217 |
| US Patents: | 10 |
| Tradenames: | 97 |
| Ingredients: | 85 |
| NDAs: | 122 |
Drugs and US Patents for SANOFI AVENTIS US
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | TACE | chlorotrianisene | CAPSULE;ORAL | 008102-004 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | TACE | chlorotrianisene | CAPSULE;ORAL | 016235-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | PHISO-SCRUB | hexachlorophene | SPONGE;TOPICAL | 017446-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | TORNALATE | bitolterol mesylate | AEROSOL, METERED;INHALATION | 018770-001 | Dec 28, 1984 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | PRESAMINE | imipramine hydrochloride | TABLET;ORAL | 011836-006 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | LASIX | furosemide | SOLUTION;ORAL | 017688-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | AVALIDE | hydrochlorothiazide; irbesartan | TABLET;ORAL | 020758-001 | Sep 30, 1997 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SANOFI AVENTIS US
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | AVAPRO | irbesartan | TABLET;ORAL | 020757-003 | Sep 30, 1997 | 5,270,317*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | NEGGRAM | nalidixic acid | TABLET;ORAL | 014214-005 | Approved Prior to Jan 1, 1982 | 3,590,036 | ⤷ Try a Trial |
| Sanofi Aventis Us | ELOXATIN | oxaliplatin | INJECTABLE;INTRAVENOUS | 021759-003 | Nov 17, 2006 | 5,290,961*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | TORNALATE | bitolterol mesylate | AEROSOL, METERED;INHALATION | 018770-001 | Dec 28, 1984 | 4,138,581 | ⤷ Try a Trial |
| Sanofi Aventis Us | ELOXATIN | oxaliplatin | INJECTABLE;INTRAVENOUS | 021759-002 | Jan 31, 2005 | 5,290,961*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | TORNALATE | bitolterol mesylate | AEROSOL, METERED;INHALATION | 018770-001 | Dec 28, 1984 | 4,336,400 | ⤷ Try a Trial |
| Sanofi Aventis Us | LASIX | furosemide | INJECTABLE;INJECTION | 016363-001 | Approved Prior to Jan 1, 1982 | 4,324,779 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANOFI AVENTIS US drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2013-07-01 |
| ➤ Subscribe | Injection | 5 mg/mL, 10 mL and 20 mL vials | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 200 mg/40 mL | ➤ Subscribe | 2007-07-16 |
| ➤ Subscribe | Injection | 100 mg/mL, 3 mL vials | ➤ Subscribe | 2006-12-07 |
| ➤ Subscribe | Extended-release Tablets | 12.5 mg | ➤ Subscribe | 2006-01-19 |
| ➤ Subscribe | Tablets | 150 mg/12.5 mg and 300 mg/12.5 mg | ➤ Subscribe | 2004-11-10 |
| ➤ Subscribe | Tablets | 75 mg, 150 mg and 300 mg | ➤ Subscribe | 2004-05-25 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-03-04 |
| ➤ Subscribe | Injection | 40 mg/mL, 0.5 mL and 2 mL vials | ➤ Subscribe | 2009-06-30 |
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 5 mg/mL, 40 mL vial | ➤ Subscribe | 2011-03-23 |
| ➤ Subscribe | Extended-release Tablets | 6.25 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Tablets | 7 mg and 14 mg | ➤ Subscribe | 2016-09-12 |
| ➤ Subscribe | Tablets | 300 mg/25 mg | ➤ Subscribe | 2006-06-06 |
International Patents for SANOFI AVENTIS US Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Japan | 2013509394 | ⤷ Try a Trial |
| Japan | 5566466 | ⤷ Try a Trial |
| World Intellectual Property Organization (WIPO) | 2005028462 | ⤷ Try a Trial |
| Argentina | 078383 | ⤷ Try a Trial |
| Panama | 8542901 | ⤷ Try a Trial |
| Portugal | 2477611 | ⤷ Try a Trial |
| Russian Federation | 2015151938 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SANOFI AVENTIS US Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1381356 | C300644 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: TERIFLUONOMIDE, ITS STEROISOMER AND PHARAMCEUTICALLY ACCEPTABLE SALTS THEREOF; REGISTRATION NO/DATE: EU/1/13/838/001-005 20130826 |
| 1381356 | 92366 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMIDE,SON STEREOISOMERE ET LEURS SELS PHARMACEUTIQUEMENT ACCEPTABLES |
| 2344130 | 132017000093887 | Italy | ⤷ Try a Trial | PRODUCT NAME: OZENOXACIN(DUBINE); AUTHORISATION NUMBER(S) AND DATE(S): ES/H/414/01/DC, 20170519;045237017, 20170711 |
| 0281459 | 9890035 | Sweden | ⤷ Try a Trial | PRODUCT NAME: PLAVIX-CLOPIDOGREL; REG. NO/DATE: EU/1/98/069/001 19980715 |
| 2768484 | 132019000000144 | Italy | ⤷ Try a Trial | PRODUCT NAME: DAUNORUBICINA E CITARABINA(VYXEOS); AUTHORISATION NUMBER(S) AND DATE(S): EU/1/18/1308, 20180827 |
| 1381356 | 8/2014 | Austria | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMID, SEINE STEREOISOMERE AND PHARMAZEUTISCH ANNEHMBARE SALZE DAVON; REGISTRATION NO/DATE: EU/1/13/838 20130826 |
| 1381356 | CR 2014 00005 | Denmark | ⤷ Try a Trial | PRODUCT NAME: TERIFLUNOMID, DETS STEREOISOMER OG FARMACEUTISK ACCEPTABLE SALTE DERAF; REG. NO/DATE: EU/1/13/838/001-005 20130826 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.