SANOFI AVENTIS US Company Profile
✉ Email this page to a colleague
What is the competitive landscape for SANOFI AVENTIS US, and when can generic versions of SANOFI AVENTIS US drugs launch?
SANOFI AVENTIS US has one hundred and twenty-two approved drugs.
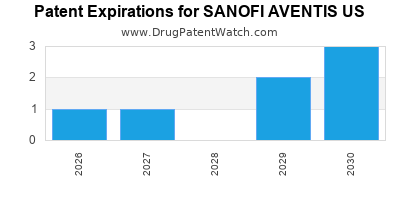
There are ten US patents protecting SANOFI AVENTIS US drugs.
There are two hundred and seventeen patent family members on SANOFI AVENTIS US drugs in fifty-two countries and one hundred and fifteen supplementary protection certificates in nineteen countries.
Summary for SANOFI AVENTIS US
| International Patents: | 217 |
| US Patents: | 10 |
| Tradenames: | 97 |
| Ingredients: | 85 |
| NDAs: | 122 |
Drugs and US Patents for SANOFI AVENTIS US
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | FERRLECIT | ferric oxyhydroxide | INJECTABLE;INJECTION | 020955-001 | Feb 18, 1999 | AB | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Sanofi Aventis Us | TALACEN | acetaminophen; pentazocine hydrochloride | TABLET;ORAL | 018458-001 | Sep 23, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | ISUPREL | isoproterenol hydrochloride | TABLET;RECTAL, SUBLINGUAL | 006328-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Sanofi Aventis Us | DORIDEN | glutethimide | CAPSULE;ORAL | 009519-008 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for SANOFI AVENTIS US
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Sanofi Aventis Us | AMARYL | glimepiride | TABLET;ORAL | 020496-003 | Nov 30, 1995 | 4,379,785*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | LOVENOX (PRESERVATIVE FREE) | enoxaparin sodium | INJECTABLE;SUBCUTANEOUS | 020164-003 | Mar 27, 1998 | 4,692,435 | ⤷ Try a Trial |
| Sanofi Aventis Us | ELOXATIN | oxaliplatin | INJECTABLE;INTRAVENOUS | 021492-002 | Aug 9, 2002 | 5,290,961*PED | ⤷ Try a Trial |
| Sanofi Aventis Us | JEVTANA KIT | cabazitaxel | SOLUTION;INTRAVENOUS | 201023-001 | Jun 17, 2010 | 5,438,072 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for SANOFI AVENTIS US drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | For Injection | 50 mg/vial and 100 mg/vial | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 5 mg/mL, 40 mL vial | ➤ Subscribe | 2011-03-23 |
| ➤ Subscribe | Extended-release Tablets | 6.25 mg | ➤ Subscribe | 2006-02-24 |
| ➤ Subscribe | Tablets | 7 mg and 14 mg | ➤ Subscribe | 2016-09-12 |
| ➤ Subscribe | Tablets | 300 mg/25 mg | ➤ Subscribe | 2006-06-06 |
| ➤ Subscribe | Tablets | 400 mg | ➤ Subscribe | 2013-07-01 |
| ➤ Subscribe | Injection | 5 mg/mL, 10 mL and 20 mL vials | ➤ Subscribe | 2007-02-09 |
| ➤ Subscribe | Injection | 200 mg/40 mL | ➤ Subscribe | 2007-07-16 |
| ➤ Subscribe | Injection | 100 mg/mL, 3 mL vials | ➤ Subscribe | 2006-12-07 |
| ➤ Subscribe | Extended-release Tablets | 12.5 mg | ➤ Subscribe | 2006-01-19 |
| ➤ Subscribe | Tablets | 150 mg/12.5 mg and 300 mg/12.5 mg | ➤ Subscribe | 2004-11-10 |
| ➤ Subscribe | Tablets | 75 mg, 150 mg and 300 mg | ➤ Subscribe | 2004-05-25 |
| ➤ Subscribe | Tablets | 300 mg | ➤ Subscribe | 2009-03-04 |
| ➤ Subscribe | Injection | 40 mg/mL, 0.5 mL and 2 mL vials | ➤ Subscribe | 2009-06-30 |
International Patents for SANOFI AVENTIS US Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 078383 | ⤷ Try a Trial |
| Hungary | E032963 | ⤷ Try a Trial |
| El Salvador | 2012004192 | ⤷ Try a Trial |
| Canada | 2721491 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for SANOFI AVENTIS US Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1667986 | 2013/021 | Ireland | ⤷ Try a Trial | PRODUCT NAME: CABAZITAXEL ACETONE SOLVATE; REGISTRATION NO/DATE: EU/1/11/676/001 20110317 |
| 1007030 | 10C0031 | France | ⤷ Try a Trial | PRODUCT NAME: DRONEDARONE; REGISTRATION NO/DATE IN FRANCE: EU/1/09/591/001 DU 20091126; REGISTRATION NO/DATE AT EEC: EU/1/09/591/001-004 DU 20091126 |
| 2236132 | C300714 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: ZOLPIDEM EN FARMACEUTISCH AANVAARDBARE ZOUTEN DAARVAN; NAT. REGISTRATION NO/DATE: RVG 108438 - 439 20130624; FIRST REGISTRATION: BE424286BE424295 2012180718 |
| 2768484 | 122019000091 | Germany | ⤷ Try a Trial | PRODUCT NAME: KOMBINATION VON DAUNORUBICIN UND CYTARABIN; REGISTRATION NO/DATE: EU/1/18/1308 20180823 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.