Pfizer Company Profile
✉ Email this page to a colleague
What is the competitive landscape for PFIZER, and when can generic versions of PFIZER drugs launch?
PFIZER has one hundred and ninety-four approved drugs.
There are forty-eight US patents protecting PFIZER drugs. There is one tentative approval on PFIZER drugs.
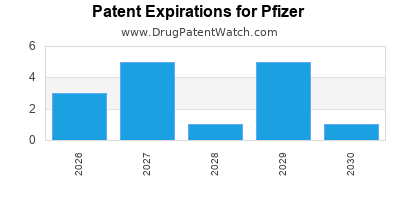
There are one thousand and twenty-two patent family members on PFIZER drugs in seventy countries and two hundred and fifty-nine supplementary protection certificates in nineteen countries.
Summary for Pfizer
| International Patents: | 1022 |
| US Patents: | 48 |
| Tradenames: | 159 |
| Ingredients: | 130 |
| NDAs: | 194 |
| Drug Master File Entries: | 3 |
| Patent Litigation for Pfizer: | See patent lawsuits for Pfizer |
| PTAB Cases with Pfizer as petitioner: | See PTAB cases with Pfizer as petitioner |
Drugs and US Patents for Pfizer
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pfizer | TROBICIN | spectinomycin hydrochloride | INJECTABLE;INJECTION | 050347-001 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pfizer Labs | DOCETAXEL | docetaxel | INJECTABLE;INJECTION | 202356-003 | Mar 13, 2014 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pfizer | ESTRING | estradiol | INSERT, EXTENDED RELEASE;VAGINAL | 020472-001 | Apr 26, 1996 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pfizer | PFIZERPEN VK | penicillin v potassium | TABLET;ORAL | 061836-002 | Approved Prior to Jan 1, 1982 | DISCN | No | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pfizer | CALAN SR | verapamil hydrochloride | TABLET, EXTENDED RELEASE;ORAL | 019152-002 | Dec 15, 1989 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Pfizer | SINEQUAN | doxepin hydrochloride | CAPSULE;ORAL | 016798-005 | Approved Prior to Jan 1, 1982 | DISCN | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Pfizer
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Pfizer | CAVERJECT | alprostadil | INJECTABLE;INJECTION | 020379-004 | May 19, 1997 | 5,741,523 | ⤷ Try a Trial |
| Pfizer | ZINECARD | dexrazoxane hydrochloride | INJECTABLE;INJECTION | 020212-001 | May 26, 1995 | 4,275,063 | ⤷ Try a Trial |
| Pfizer | TIKOSYN | dofetilide | CAPSULE;ORAL | 020931-002 | Oct 1, 1999 | 6,124,363 | ⤷ Try a Trial |
| Pfizer Pharms | ACCUPRIL | quinapril hydrochloride | TABLET;ORAL | 019885-002 | Nov 19, 1991 | 4,344,949*PED | ⤷ Try a Trial |
| Pfizer | MINIZIDE | polythiazide; prazosin hydrochloride | CAPSULE;ORAL | 017986-001 | Approved Prior to Jan 1, 1982 | 3,663,706 | ⤷ Try a Trial |
| Pfizer | NICOTROL | nicotine | INHALANT;ORAL | 020714-001 | May 2, 1997 | 4,800,903 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for PFIZER drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 50 mg/0.2 mg | ➤ Subscribe | 2009-06-29 |
| ➤ Subscribe | Extended-release Tablets | 11 mg | ➤ Subscribe | 2016-11-07 |
| ➤ Subscribe | Capsules | 20 mg, 40 mg, 60 mg and 80 mg | ➤ Subscribe | 2005-02-07 |
| ➤ Subscribe | Capsules | 75 mg, 100 mg and 125 mg | ➤ Subscribe | 2019-02-04 |
| ➤ Subscribe | Capsules | 0.125 mg, 0.25 mg, and 0.5 mg | ➤ Subscribe | 2014-05-01 |
| ➤ Subscribe | Tablets | 600 mg | ➤ Subscribe | 2005-12-21 |
| ➤ Subscribe | Injection | 2 mg/mL, 300 mL bag | ➤ Subscribe | 2009-09-01 |
| ➤ Subscribe | Tablets | 1 g | ➤ Subscribe | 2005-08-23 |
| ➤ Subscribe | Delayed-release Tablets | 75 mg/0.2 mg | ➤ Subscribe | 2008-11-28 |
| ➤ Subscribe | Extended-release Tablets | 4 mg and 8 mg | ➤ Subscribe | 2012-10-31 |
| ➤ Subscribe | Injection | 20 mg/mL, 2 mL and 5 mL vials | ➤ Subscribe | 2004-07-26 |
| ➤ Subscribe | Capsules | 5 mg and 10 mg | ➤ Subscribe | 2005-06-21 |
| ➤ Subscribe | For Injection | 500 mg/vial | ➤ Subscribe | 2011-06-17 |
| ➤ Subscribe | Oral Suspension | 100 mg/5 mL | ➤ Subscribe | 2009-08-03 |
| ➤ Subscribe | Injection | 2 mg/mL, 100 mL bag | ➤ Subscribe | 2009-12-29 |
| ➤ Subscribe | Tablets | 5 mg | ➤ Subscribe | 2016-11-07 |
International Patents for Pfizer Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Argentina | 083686 | ⤷ Try a Trial |
| Taiwan | 201422617 | ⤷ Try a Trial |
| Serbia | 63714 | ⤷ Try a Trial |
| New Zealand | 537315 | ⤷ Try a Trial |
| Japan | 6041823 | ⤷ Try a Trial |
| Taiwan | 200427453 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Pfizer Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 2958921 | 2022C/505 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ABROCITINIB, OF EEN FARMACEUTISCH AANVAARDBAAR ZOUT DAARVAN; AUTHORISATION NUMBER AND DATE: EU/1/21/1593 20211210 |
| 2488512 | C02488512/01 | Switzerland | ⤷ Try a Trial | PRODUCT NAME: RIMEGEPANT; REGISTRATION NO/DATE: SWISSMEDIC-ZULASSUNG 69035 18.10.2023 |
| 0770388 | 2009/012 | Ireland | ⤷ Try a Trial | PRODUCT NAME: QLAIRA-ESTRADIOL VALERATE/DIENOGEST; NAT REGISTRATION NO/DATE: PA1410/58/1 20090109; FIRST REGISTRATION NO/DATE: BE327792 20081103 |
| 2822953 | 301006 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LORLATINIB, DESGEWENST IN DE VORM VAN EEN FARMACEUTISCH AANVAARDBAAR ZOUT; REGISTRATION NO/DATE: EU/1/19/1355 20190508 |
| 1666481 | 2017/036 | Ireland | ⤷ Try a Trial | PRODUCT NAME: TOFACITINIB, OPTIONALLY IN THE FORM OF A PHARMACEUTICALLY ACCEPTABLE SALT, INCLUDING THE CITRATE SALT; REGISTRATION NO/DATE: EU/1/17/1178/001 EU/1/17/1178/004 20170322 |
| 2767537 | 122019000108 | Germany | ⤷ Try a Trial | PRODUCT NAME: TALAZOPARIB ODER PHARMAZEUTISCH VERTRAEGLICHES SALZ DAVON; REGISTRATION NO/DATE: EU/1/19/1377 20190620 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.