OTSUKA Company Profile
✉ Email this page to a colleague
What is the competitive landscape for OTSUKA, and what generic alternatives to OTSUKA drugs are available?
OTSUKA has seventeen approved drugs.
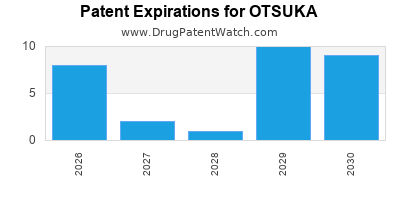
There are fifty-six US patents protecting OTSUKA drugs.
There are one thousand and seventeen patent family members on OTSUKA drugs in fifty-four countries and forty-nine supplementary protection certificates in sixteen countries.
Summary for OTSUKA
| International Patents: | 1017 |
| US Patents: | 56 |
| Tradenames: | 15 |
| Ingredients: | 10 |
| NDAs: | 17 |
| Patent Litigation for OTSUKA: | See patent lawsuits for OTSUKA |
Drugs and US Patents for OTSUKA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-003 | Sep 29, 2014 | RX | Yes | No | 11,154,553 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-005 | Nov 13, 2017 | RX | Yes | No | 9,149,577 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-004 | Nov 13, 2017 | RX | Yes | No | 8,114,021 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka | ABILIFY ASIMTUFII | aripiprazole | SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 217006-001 | Apr 27, 2023 | RX | Yes | No | 11,097,007 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Otsuka | ABILIFY | aripiprazole | TABLET;ORAL | 021436-002 | Nov 15, 2002 | AB | RX | Yes | No | 9,125,939 | ⤷ Try a Trial | ⤷ Try a Trial | |||
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-004 | Sep 29, 2014 | RX | Yes | No | 11,154,553 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Otsuka | REXULTI | brexpiprazole | TABLET;ORAL | 205422-004 | Jul 10, 2015 | AB | RX | Yes | Yes | 7,888,362 | ⤷ Try a Trial | Y | Y | ⤷ Try a Trial | |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for OTSUKA
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Otsuka | ABILIFY | aripiprazole | TABLET;ORAL | 021436-001 | Nov 15, 2002 | 8,642,760*PED | ⤷ Try a Trial |
| Otsuka Pharm Co Ltd | ABILIFY MAINTENA KIT | aripiprazole | FOR SUSPENSION, EXTENDED RELEASE;INTRAMUSCULAR | 202971-001 | Feb 28, 2013 | 8,759,351 | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET, ORALLY DISINTEGRATING;ORAL | 021729-004 | Jun 7, 2006 | 9,359,302 | ⤷ Try a Trial |
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-002 | Nov 13, 2017 | 8,642,760 | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | SOLUTION;ORAL | 021713-001 | Dec 10, 2004 | 5,006,528*PED | ⤷ Try a Trial |
| Otsuka | ABILIFY MYCITE KIT | aripiprazole | TABLET;ORAL | 207202-004 | Nov 13, 2017 | 9,359,302 | ⤷ Try a Trial |
| Otsuka | ABILIFY | aripiprazole | TABLET, ORALLY DISINTEGRATING;ORAL | 021729-002 | Jun 7, 2006 | 9,387,182 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for OTSUKA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Tablets | 2 mg, 5 mg, 10 mg, 15 mg, 20 mg and 30 mg | ➤ Subscribe | 2006-11-15 |
| ➤ Subscribe | Tablets | 15 mg and 30 mg | ➤ Subscribe | 2013-09-23 |
| ➤ Subscribe | Injection | 6 mg/mL | ➤ Subscribe | 2012-12-26 |
| ➤ Subscribe | Oral Solution | 1 mg/mL | ➤ Subscribe | 2007-12-20 |
| ➤ Subscribe | Orally Disintegrating Tablets | 10 mg, 15 mg, 20 mg and 30 mg | ➤ Subscribe | 2006-11-15 |
| ➤ Subscribe | Tablets | 60 mg | ➤ Subscribe | 2018-03-26 |
International Patents for OTSUKA Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Cyprus | 1112496 | ⤷ Try a Trial |
| Portugal | 3235491 | ⤷ Try a Trial |
| South Korea | 20140066996 | ⤷ Try a Trial |
| Hungary | S2300045 | ⤷ Try a Trial |
| Norway | 336264 | ⤷ Try a Trial |
| Israel | 173441 | ⤷ Try a Trial |
| India | 508CHN2014 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for OTSUKA Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0450097 | 09C0049 | France | ⤷ Try a Trial | PRODUCT NAME: TOLVAPTAN, EVENTUELLEMENT SOUS FORME DE SEL; REGISTRATION NO/DATE: EU/1/09/539/001 20090803 |
| 2207786 | CA 2023 00037 | Denmark | ⤷ Try a Trial | PRODUCT NAME: CEDAZURIDIN ELLER ET FARMACEUTISK ACCEPTABELT SALT DERAF; REG. NO/DATE: EU/1/23/1756 20230918 |
| 2207786 | LUC00326 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION COMPRENANT: DE LA CEDAZURIDINE OU UN DE SES SELS PHARMACEUTIQUEMENT ACCEPTABLES; ET DE LA DECITABINE; AUTHORISATION NUMBER AND DATE: EU/1/23/1756 20230913 |
| 2207786 | PA2023538 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: CEDAZURIDINAS ARBA FARMACINIU POZIURIU PRIIMTINA JO DRUSKA; REGISTRATION NO/DATE: EU/1/23/1756 20230915 |
| 1869025 | CR 2018 00028 | Denmark | ⤷ Try a Trial | PRODUCT NAME: BREXPIPRAZOL ELLER ET SALT DERAF; REG. NO/DATE: EU/1/18/1294/001-006 20180727 |
| 1675573 | C20140014 00322 | Estonia | ⤷ Try a Trial | PRODUCT NAME: ARIPIPRASOOL;REG NO/DATE: K(2013)8163 (LOPLIK) 19.11.2013 |
| 0451216 | 99C0028 | Belgium | ⤷ Try a Trial | PRODUCT NAME: GREPAFLOXACINE; CHLORHYDRATE SESQUIHYDRATE; NAT. REGISTRATION NO/DATE: NL 23 373 19990525; FIRST REGISTRATION: DE - 4062.00.00 19970731 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.