Mayne Pharma Company Profile
✉ Email this page to a colleague
What is the competitive landscape for MAYNE PHARMA, and what generic alternatives to MAYNE PHARMA drugs are available?
MAYNE PHARMA has fifteen approved drugs.
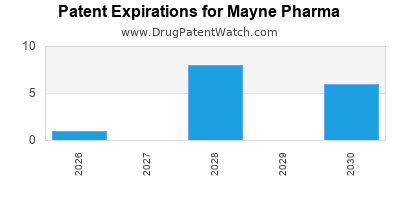
There are seventy-eight US patents protecting MAYNE PHARMA drugs. There is one tentative approval on MAYNE PHARMA drugs.
There are four hundred and twenty-five patent family members on MAYNE PHARMA drugs in forty-nine countries and sixty supplementary protection certificates in fourteen countries.
Summary for Mayne Pharma
| International Patents: | 425 |
| US Patents: | 78 |
| Tradenames: | 18 |
| Ingredients: | 14 |
| NDAs: | 15 |
Drugs and US Patents for Mayne Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mayne Pharma | IMVEXXY | estradiol | INSERT;VAGINAL | 208564-002 | May 29, 2018 | RX | Yes | Yes | 10,668,082 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mayne Pharma | IMVEXXY | estradiol | INSERT;VAGINAL | 208564-002 | May 29, 2018 | RX | Yes | Yes | 11,123,283 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mayne Pharma | RHOFADE | oxymetazoline hydrochloride | CREAM;TOPICAL | 208552-001 | Jan 18, 2017 | RX | Yes | Yes | 8,420,688 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mayne Pharma | ANNOVERA | ethinyl estradiol; segesterone acetate | RING;VAGINAL | 209627-001 | Aug 10, 2018 | RX | Yes | Yes | 10,632,066 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mayne Pharma | DORYX | doxycycline hyclate | TABLET, DELAYED RELEASE;ORAL | 050795-004 | Apr 11, 2013 | RX | Yes | No | 8,715,724 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Mayne Pharma | IMVEXXY | estradiol | INSERT;VAGINAL | 208564-002 | May 29, 2018 | RX | Yes | Yes | 10,398,708 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Mayne Pharma | NEXTSTELLIS | drospirenone; estetrol | TABLET;ORAL | 214154-001 | Apr 15, 2021 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for Mayne Pharma
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Mayne Pharma | DORYX | doxycycline hyclate | TABLET, DELAYED RELEASE;ORAL | 050795-003 | Jun 20, 2008 | 6,958,161 | ⤷ Try a Trial |
| Mayne Pharma | DORYX | doxycycline hyclate | TABLET, DELAYED RELEASE;ORAL | 050795-005 | Apr 11, 2013 | 6,958,161 | ⤷ Try a Trial |
| Mayne Pharma | TOLSURA | itraconazole | CAPSULE;ORAL | 208901-001 | Dec 11, 2018 | 8,771,739 | ⤷ Try a Trial |
| Mayne Pharma | RHOFADE | oxymetazoline hydrochloride | CREAM;TOPICAL | 208552-001 | Jan 18, 2017 | 8,815,929 | ⤷ Try a Trial |
| Mayne Pharma | DORYX MPC | doxycycline hyclate | TABLET, DELAYED RELEASE;ORAL | 050795-007 | May 20, 2016 | 6,958,161 | ⤷ Try a Trial |
| Mayne Pharma | DORYX MPC | doxycycline hyclate | TABLET, DELAYED RELEASE;ORAL | 050795-008 | May 20, 2016 | 6,958,161 | ⤷ Try a Trial |
| Mayne Pharma | DORYX | doxycycline hyclate | TABLET, DELAYED RELEASE;ORAL | 050795-002 | May 6, 2005 | 6,958,161 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
Paragraph IV (Patent) Challenges for MAYNE PHARMA drugs
| Drugname | Dosage | Strength | Tradename | Submissiondate |
|---|---|---|---|---|
| ➤ Subscribe | Delayed-release Tablets | 200 mg | ➤ Subscribe | 2013-08-12 |
| ➤ Subscribe | Delayed-release Tablets | 80 mg | ➤ Subscribe | 2015-07-01 |
| ➤ Subscribe | Delayed-release Tablets | 60 mg and 120 mg | ➤ Subscribe | 2017-09-28 |
| ➤ Subscribe | Delayed-release Tablets | 150 mg | ➤ Subscribe | 2008-12-19 |
| ➤ Subscribe | Delayed-release Tablets | 200 mg | ➤ Subscribe | 2014-05-19 |
| ➤ Subscribe | Delayed-release Tablets | 50 mg | ➤ Subscribe | 2015-11-05 |
International Patents for Mayne Pharma Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Germany | 60223795 | ⤷ Try a Trial |
| Japan | 2017509630 | ⤷ Try a Trial |
| Russian Federation | 2015100533 | ⤷ Try a Trial |
| Japan | 6656215 | ⤷ Try a Trial |
| South Korea | 101725196 | ⤷ Try a Trial |
| European Patent Office | 1446128 | ⤷ Try a Trial |
| Croatia | P20211968 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for Mayne Pharma Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 0398460 | 04C0022 | France | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL ANHYDRE DROSPIRENONE; REGISTRATION NO/DATE IN FRANCE: NL 28661 DU 20040316; REGISTRATION NO/DATE AT EEC: RVG 27505 DU 20021211 |
| 3701944 | PA2022508 | Lithuania | ⤷ Try a Trial | PRODUCT NAME: DROSPIRENONAS DERINYJE SU ESTETROLIU; REGISTRATION NO/DATE: EU/1/21/1547 20210519 |
| 1453521 | 300814 | Netherlands | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL EN ETHINYLESTRADIOL; NATIONAL REGISTRATION NO/DATE: RVG 117453 20151211; FIRST REGISTRATION: SK 17/0017/15-S 20150211 |
| 0770388 | 09C0018 | France | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL VALERATE; DIENOGEST; NAT. REGISTRATION NO/DATE: NL35170 20081210; FIRST REGISTRATION: BE327792 20081103 |
| 0975367 | 122011000009 | Germany | ⤷ Try a Trial | PRODUCT NAME: FENTANYL IN ALLEN DEM SCHUTZ DES GRUNDPATENTS UNTERLIEGENDEN FORMEN; REGISTRATION NO/DATE: EU/1/10/644/001-004 20100831 |
| 2782584 | 21C1058 | France | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), Y COMPRIS SOUS FORME HEMIHYDRATEE, ET DE LA PROGESTERONE; NAT. REGISTRATION NO/DATE: NL51886 20210421; FIRST REGISTRATION: BE - BE582231 20210406 |
| 0402407 | 97C0005 | Belgium | ⤷ Try a Trial | PRODUCT NAME: ESTRADIOL HEMIHYDRAAT; NAT. REGISTRATION NO/DATE: 298 IS 190 F 15 19960806; FIRST REGISTRATION: GB PL/0053/0241 19950711 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.