MILLICENT Company Profile
✉ Email this page to a colleague
What is the competitive landscape for MILLICENT, and when can generic versions of MILLICENT drugs launch?
MILLICENT has two approved drugs.
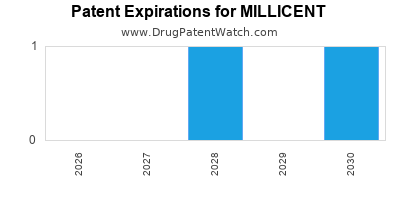
There are three US patents protecting MILLICENT drugs.
There are fifty-six patent family members on MILLICENT drugs in thirty-one countries and forty-one supplementary protection certificates in fourteen countries.
Drugs and US Patents for MILLICENT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | TE | Type | RLD | RS | Patent No. | Patent Expiration | Product | Substance | Delist Req. | Exclusivity Expiration |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Millicent | INTRAROSA | prasterone | INSERT;VAGINAL | 208470-001 | Nov 16, 2016 | RX | Yes | Yes | 8,629,129 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Millicent | FEMRING | estradiol acetate | INSERT, EXTENDED RELEASE;VAGINAL | 021367-001 | Mar 20, 2003 | RX | Yes | No | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| Millicent | INTRAROSA | prasterone | INSERT;VAGINAL | 208470-001 | Nov 16, 2016 | RX | Yes | Yes | 8,957,054 | ⤷ Try a Trial | ⤷ Try a Trial | ||||
| Millicent | INTRAROSA | prasterone | INSERT;VAGINAL | 208470-001 | Nov 16, 2016 | RX | Yes | Yes | 8,268,806 | ⤷ Try a Trial | Y | ⤷ Try a Trial | |||
| Millicent | FEMRING | estradiol acetate | INSERT, EXTENDED RELEASE;VAGINAL | 021367-002 | Mar 20, 2003 | RX | Yes | Yes | ⤷ Try a Trial | ⤷ Try a Trial | |||||
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >TE | >Type | >RLD | >RS | >Patent No. | >Patent Expiration | >Product | >Substance | >Delist Req. | >Exclusivity Expiration |
Expired US Patents for MILLICENT
| Applicant | Tradename | Generic Name | Dosage | NDA | Approval Date | Patent No. | Patent Expiration |
|---|---|---|---|---|---|---|---|
| Millicent | FEMRING | estradiol acetate | INSERT, EXTENDED RELEASE;VAGINAL | 021367-001 | Mar 20, 2003 | 5,855,906 | ⤷ Try a Trial |
| Millicent | FEMRING | estradiol acetate | INSERT, EXTENDED RELEASE;VAGINAL | 021367-002 | Mar 20, 2003 | 5,855,906 | ⤷ Try a Trial |
| >Applicant | >Tradename | >Generic Name | >Dosage | >NDA | >Approval Date | >Patent No. | >Patent Expiration |
International Patents for MILLICENT Drugs
| Country | Patent Number | Estimated Expiration |
|---|---|---|
| Chile | 2008002362 | ⤷ Try a Trial |
| Taiwan | 201212922 | ⤷ Try a Trial |
| South Korea | 20100061671 | ⤷ Try a Trial |
| Taiwan | I609688 | ⤷ Try a Trial |
| Israel | 203747 | ⤷ Try a Trial |
| >Country | >Patent Number | >Estimated Expiration |
Supplementary Protection Certificates for MILLICENT Drugs
| Patent Number | Supplementary Protection Certificate | SPC Country | SPC Expiration | SPC Description |
|---|---|---|---|---|
| 1380301 | CA 2009 00017 | Denmark | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL (SOM BETADEXCLATHRAT) OG DROSPIRENON; NAT. REG. NO/DATE: 42417 (DK) 20080619; FIRST REG. NO/DATE: NL 33842 20070629 |
| 2782584 | LUC00245 | Luxembourg | ⤷ Try a Trial | PRODUCT NAME: COMPOSITION CONTENANT A LA FOIS DE L'ESTRADIOL (17SS-ESTRADIOL), EVENTUELLEMENT SOUS FORME D'UN SEL, HYDRATE OU SOLVATE PHARMACEUTIQUEMENT ACCEPTABLE DE CELUI-CI (Y COMPRIS SOUS FORME HEMIHYDRATEE), ET DE LA PROGESTERONE; AUTHORISATION NUMBER AND DATE: BE582231 20210701 |
| 1453521 | 15C0050 | France | ⤷ Try a Trial | PRODUCT NAME: ETHINYLESTRADIOL ET MELANGE DE LEVONORGESTREL ET ETHINYLESTRADIOL; NAT. REGISTRATION NO/DATE: NL 42237 20150320; FIRST REGISTRATION: SK - 17/0017/15-S 20150129 |
| 1453521 | CA 2016 00016 | Denmark | ⤷ Try a Trial | PRODUCT NAME: LEVONORGESTREL OG ETHINYLOESTRADIOL; NAT. REG. NO/DATE: 56336 20151105; FIRST REG. NO/DATE: SK 17/0017/15-S 20150211 |
| 1453521 | C201630040 | Spain | ⤷ Try a Trial | PRODUCT NAME: ETINILESTRADIOL Y MEZCLA DE LEVONORGESTREL Y ETINILESTRADIOL; NATIONAL AUTHORISATION NUMBER: 80340; DATE OF AUTHORISATION: 20160122; NUMBER OF FIRST AUTHORISATION IN EUROPEAN ECONOMIC AREA (EEA): 17/0017/15-S; DATE OF FIRST AUTHORISATION IN EEA: 20150211 |
| >Patent Number | >Supplementary Protection Certificate | >SPC Country | >SPC Expiration | >SPC Description |
Similar Applicant Names
Here is a list of applicants with similar names.